
In May, WHO announced the designation of November 17th as World Cervical Cancer Elimination Day. This day serves as an official observance to raise global awareness, encourage action, and deliver the message: “Let’s eliminate cervical cancer as a public health issue.”
On November 17, 2020, WHO released the “Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem” and had since informally referred to this date as the Day of Action. By designating it as an official campaign, WHO emphasized the need for more proactive efforts in eliminating cervical cancer. This official recognition goes beyond raising awareness—it signals to countries worldwide that it is time to translate the three core targets—prevention, early detection, and expanded access to treatment—into concrete actions to achieve the 2030 elimination goals.
Cervical Cancer: A Treatable Cancer

Source: shutterstock
WHO, along with other international health organizations and the medical community, considers cervical cancer a treatable cancer. This is because it can often be detected early, and both preventive and therapeutic measures are well established. Over 95% of cervical cancer cases are caused by HPV infection, which is preventable through vaccination. Additionally, cervical cancer progresses through a precancerous stage before becoming invasive, allowing for early detection through screening and effective treatment that can lead to complete cure.
Cervical cancer is highly treatable when detected early through regular screening, which is why WHO set a target for over 70% of women at ages 35 and 45 to be screened as part of its elimination strategy. In the early stages, non-surgical treatments can achieve complete cure, and even in more advanced cases, effective treatment is possible. Because it is preventable, detectable at an early stage, and treatable, WHO became the first organization to designate cervical cancer as a public health problem that can be eliminated.
The Key to Elimination: The Importance of Diagnosis

Source: shutterstock
The key to eliminating cervical cancer is action. Prevention, diagnosis, and treatment must be implemented in an integrated and high-quality manner, forming the core of WHO’s 90-70-90 targets. In particular, cervical cancer diagnosis, which corresponds to the 70% screening target, plays a critical role in saving lives today and in the near future.
Cervical cancer progresses through a precancerous stage that can last from several years to decades before becoming invasive. If abnormalities are detected during this stage through regular screening, simple procedures can achieve a complete cure, effectively preventing progression to cancer.
For adult women who have not received the HPV vaccine, screening remains the only public health defense for preventing and detecting cervical cancer early. This is why WHO set a target for 70% of women aged 35–45 to undergo regular screening. Early detection at the precancerous stage is not only cost-effective—avoiding the high expenses associated with treatment after cancer has progressed—but also reduces the burden on patients by allowing for simple and effective interventions.
Noul Improving the Cervical Cancer Diagnostic Environment

Despite these efforts, cervical cancer remains the fourth leading cause of cancer death among women worldwide, claiming the lives of countless women. The main challenge is limited access to high-quality healthcare: approximately 90% of cervical cancer deaths occur in low- and middle-income countries (LMICs). In many of these regions, national cervical cancer screening programs are inadequate or underfunded. Additionally, women often face restricted access to quality healthcare, and there is a shortage of skilled medical personnel and high-performance infrastructure needed to accurately analyze samples. These factors create significant environmental barriers to receiving high-quality cervical cancer screening.
Noul recognizes that a key challenge in eliminating cervical cancer lies in bridging the gap in diagnostic accessibility. To address this, the company is leveraging innovative technologies to expand access to high-quality diagnostics, aiming to advance global health equity so that women worldwide can benefit from reliable cervical cancer screening. In practice, Noul has already conducted field evaluations and collaborations for miLab™ CER in various countries across Europe and Latin America, establishing itself as a practical partner in the global fight against cervical cancer.
Noul’s miLab™ CER Transforming Diagnostic Access
Source: noul
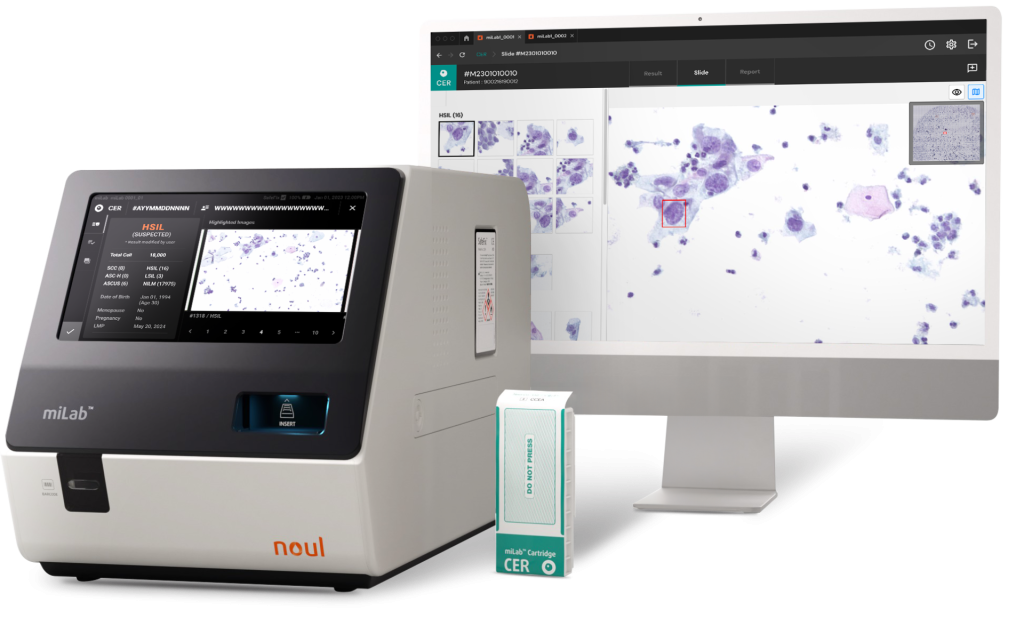
Noul’s miLab™ CER is an innovative solution designed to revolutionize access to cervical cancer diagnostics. As an all-in-one platform, it combines a portable device with a single-use cartridge, enabling diagnostics even in resource-limited settings and allowing for on-site testing.
The cartridge, equipped with Noul’s innovative technology, automates the entire workflow—from staining and imaging to analysis and diagnostic reporting—maximizing the efficiency of diagnostic professionals. Its on-device AI delivers stepwise analysis according to the Bethesda System, enabling the detection of precancerous stages.
Notably, the AI in miLab™ CER was featured in the UNITAID 2024 report as a “computer-assisted cytology system with AI-based algorithm”, officially validating its performance. As such, miLab™ CER represents a highly practical solution for addressing challenges in cervical cancer healthcare, enabling women worldwide to access high-quality diagnostics.
Learn more about miLab™ CER, the innovative solution driving the global cervical cancer elimination strategy, by clicking the link.

