Benin, a coastal nation in West Africa with a population of around 14 million, has been actively engaging in international cooperation in the healthcare sector. One of the most pressing public health challenges the country faces is malaria, with approximately 5.1 million cases reported annually.
In response, global support has been steadily increasing to help tackle this issue. Notably, NOUL’s miLab™ MAL recently entered into a public procurement agreement with the government of Benin. This partnership highlights the country’s own efforts to strengthen its diagnostic capabilities and improve healthcare infrastructure.
Benin continues to face significant challenges in diagnosing malaria effectively.
Source: Shutterstock
Currently, malaria diagnosis in Benin relies heavily on microscopy and rapid diagnostic tests (RDTs). While the public sector is relatively well-equipped with diagnostic infrastructure, enabling more systematic and accurate testing, the situation in the private sector is quite different. Low-quality RDTs are commonly used, and in many cases, treatment is prescribed without proper diagnosis. This practice often leads to serious medical complications, highlighting a critical gap in the country’s healthcare system.
Microscopy remains the most reliable method for accurately diagnosing malaria, but it requires skilled personnel and proper equipment. As a result, most people in Benin depend on the more accessible RDTs. However, these tests come with quality concerns—particularly the risk of false-negative results caused by pfhrp2 gene deletions in Plasmodium falciparum. This creates a significant diagnostic gap, making it difficult to ensure timely and accurate treatment for all patients.

Source: Shutterstock
Recognizing these challenges, Benin has been actively working to close the diagnostic gap and ensure that more of its population has access to accurate malaria infection status. The country has received strong international support and has embraced innovative technologies to improve healthcare outcomes.
The U.S. CDC and USAID have supplied Benin with around 20 million RDT kits and 20 million antimalarial treatments, significantly enhancing access to both diagnosis and care. Additionally, Korea’s NOUL has contributed by providing its on-site diagnostic device, miLab™ MAL, enabling fast and accurate malaria testing and playing a key role in curbing the spread of infection.
NOUL’s miLab™: Introducing a New Paradigm in Malaria Diagnosis for Benin
In February, NOUL made a public procurement contract with the government of Benin to supply a minimum of 219 units of its miLab™ MAL over the next three years. The Benin government has adopted NOUL’s AI-powered malaria diagnostic platform as a key solution to significantly improve diagnostic accessibility and deliver accurate results. This marks a major turning point in the country’s fight against malaria and signals a strong commitment to advancing its public health infrastructure.
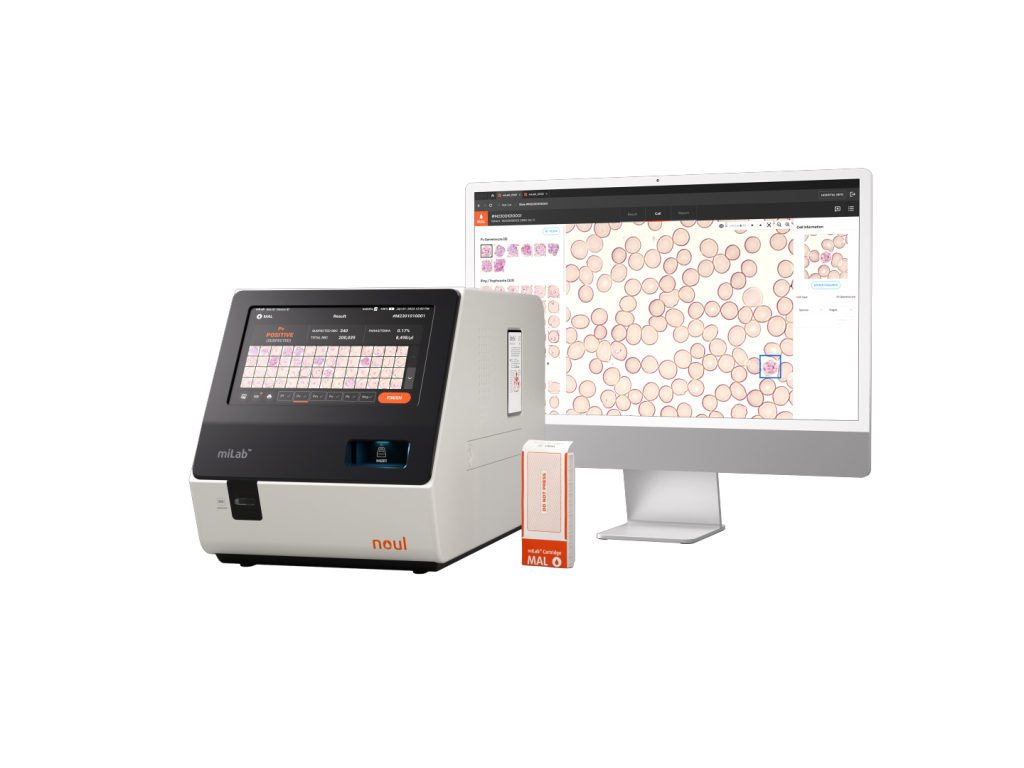
NOUL’s miLab™ MAL is a digital microscopy-based malaria diagnostic solution that integrates automated workflow, AI-powered analysis, and high-resolution digital imaging into a single compact platform. One of its key strengths lies in its ability to perform morphological analysis, enabling precise differentiation between Plasmodium falciparum and P. vivax. This helps overcome diagnostic errors commonly associated with pfhrp2/3 gene deletions in RDTs. Additionally, miLab™ MAL analyzes approximately 100,000 to 300,000 red blood cells to provide quantitative parasite density, allowing for detailed insights into the parasite’s life cycle and stage of infection.

Source: NOUL
Designed for ease of use and equipped with remote diagnostic features, miLab™ MAL can be used even in settings without fully developed medical infrastructure. This ensures that accurate diagnostics are accessible in a wide range of environments. Its selection as a scale-up stage innovation in UNITAID’s 2022 report further validates its advanced technology and high performance, reinforcing its reputation for delivering excellent diagnostic accuracy.
Diagnostic Standards Reviewed by Malaria Experts in Benin
In April, Benin officially launched a performance evaluation of NOUL’s miLab™ MAL. As an innovative technology that enables the entire malaria diagnostic process on a single compact platform, miLab™ MAL is now undergoing local validation for commercialization. The evaluation was further reinforced by the presence of key experts, including officials from Benin’s Ministry of Health, representatives from the WHO malaria program, and leaders from the National Malaria Control Program (PNLP), underscoring its significance and potential impact.

Source: NOUL
The participants included Professor Alao Jules from Benin’s Ministry of Health, Dr. Codjo from Benin’s National Malaria Control Program (PNLP), Dr. Oguo Yemi from the WHO malaria program, and Creto Kanyemba, Biomedical Research Manager at NOUL. Before the clinical evaluation began, each of them shared their insights and expectations regarding miLab™ MAL.
“Technology must be embraced across various sectors of life,” he emphasized. “In order to eradicate malaria, new technologies must be introduced into the diagnostic cycle, and miLab™ MAL is one of them.” – Professor Alao Jules (Benin Ministry of Health)
“I positively assess the potential of miLab™ technology and look forward to its future scalability and broader application.” – Dr. Codjo (National Malaria Control Program(PNLP), Benin)
“An inaccurate diagnosis can lead to serious complications, which is why reliable diagnostic tools are essential. miLab™ technology meets a satisfactory standard, and we are considering integrating it into our malaria diagnostic policies.” – Dr. Oguo Yemi (WHO Malaria Program, Health Department)
The evaluation will be conducted using approximately 400 test samples across two sites to verify the performance of miLab™ in Giemsa-stained slide microscopy readings. The goal is to assess the product’s usability and performance from the perspective of actual users, ensuring its suitability for the malaria diagnostic environment in Benin. This evaluation will lay the groundwork for future public procurement opportunities and provide a critical reference for miLab™ MAL’s market entry in Benin.
Market Potential of miLab™ MAL Proven Through Evaluation
Clinical evaluation of diagnostic devices is a crucial step for market entry. For healthcare professionals to trust and utilize diagnostic results, the device must be validated for its accuracy in real-world medical settings. Additionally, to supply the product to hospitals, clinics, and other customers, it is essential to present reliable clinical outcomes based on actual patient data.

Source: NOUL
The recent clinical evaluation of miLab™ MAL has demonstrated its potential to be a suitable solution for Benin’s malaria diagnostic environment, where the introduction of new technologies is essential.
As a result of the full automation of all testing stages, miLab™ MAL maximizes diagnostic efficiency, providing quick and accurate results that can be effectively linked to treatment, thus improving the challenges of public diagnostics. NOUL has dedicated significant effort to developing miLab™ MAL to address the ongoing global malaria epidemic and is providing solutions to help many countries, where medical access is limited, bridge the healthcare gap.
Source: NOUL
The Benin government has adopted miLab™ MAL as its national malaria diagnostic solution and is preparing for commercialization by validating its technology through field-based clinical evaluations. More details about this innovative platform, which is approaching national public procurement, can be found at the link.

