WHO considers cervical cancer a “preventable and eliminable disease” and has developed various policies to achieve this goal. In 2020, the organization officially adopted the Global Strategy for the Elimination of Cervical Cancer as a Public Health Problem at the World Health Assembly. As part of this initiative, WHO set three key targets to be achieved by 2030.
- 90% of girls are fully vaccinated with the HPV vaccine by the age of 15.
- 70% of women undergo high-performance screening by the ages of 35 and 45.
- 90% of women identified with pre-cancer or invasive cervical cancer receive appropriate treatment and management.

Source: Shutterstock
At the recent World Health Assembly, November 17 was officially designated as World Cervical Cancer Elimination Day, reaffirming the global commitment to eliminating cervical cancer. In addition, in March 2024, the World Bank, the Bill & Melinda Gates Foundation, and UNICEF pledged approximately $600 million to support cervical cancer elimination, demonstrating strong, coordinated action from major global institutions.
Despite these efforts, screening rates for cervical cancer have shown little improvement. In this post, we will explore the challenges surrounding cervical cancer screening and examine potential solutions to overcome them.
Leading Countries Actively Promoting Cervical Cancer Screening
Source: Shutterstock
As cervical cancer emerges as a major global health issue, many countries are implementing a variety of policies aimed at its elimination. The starting point for these efforts lies in encouraging regular screening. Countries such as the United Kingdom, Germany, France, and South Korea have established national-level programs to ensure more women have access to screening. Since cervical cancer is highly curable when detected early, screening plays a critical role in achieving the goal of its elimination.
Most high-income countries offer cervical cancer screening either free of charge or at a low cost, and have strengthened prevention by linking it with HPV vaccination programs. In countries like the United Kingdom, Sweden, and South Korea—where screening is actively encouraged—significant declines in both incidence and mortality rates have been observed. When governments provide screening services at no or low cost, more women can access them regardless of their economic situation. This improves healthcare accessibility and ultimately leads to increased screening rates.
Countries Where Cervical Cancer Screening Rates Remain Low

Source: Lyonstock / Shutterstock
In high-income countries, the expansion of vaccination and screening programs has led to a clear decline in both the incidence and mortality rates of cervical cancer. However, in low- and middle-income countries, vaccination coverage remains low and screening infrastructure is inadequate, resulting in persistently high cervical cancer rates. Notably, about 90% of new cases occur in these low- and middle-income countries, highlighting the urgent need for more effective cervical cancer screening strategies in these regions compared to high-income countries.
Challenges in Cervical Cancer Screening in Low- and Middle-Income Countries
- Lack of Healthcare Infrastructure: There is a severe shortage of essential facilities such as pathology laboratories, diagnostic equipment, and specialized personnel like obstetricians and cytopathologists. This results in the absence of basic infrastructure needed to conduct regular screening tests such as Pap smears and HPV DNA testing.
- Complex Workflow: The Papanicolaou staining process for cervical cytology requires highly skilled personnel. Inadequate laboratory conditions increase the risk of human error during testing.
- Lengthy Diagnostic and Treatment Processes: The extended time required from diagnosis to treatment creates a burden for patients to visit healthcare facilities, leading to poor linkage between screening and treatment.
- Lack of Government Policy and Priority: Resources are often focused on urgent health issues such as infectious diseases and maternal and child health, causing cervical cancer to be deprioritized. Government policy implementation in this area is often slow.
- Financial Barriers: The direct and indirect costs of screening, including test fees and transportation to medical facilities, impose a heavy burden. Public healthcare systems are often underdeveloped, and free screening programs are limited.
WHO emphasizes that the effective elimination of cervical cancer will remain difficult unless the healthcare challenges in low- and middle-income countries are addressed. Therefore, these countries must resolve the current issues in their healthcare systems and develop more proactive strategies to ensure that a greater number of people have access to cervical cancer screening and receive timely treatment.
Strategies to Increase Cervical Cancer Diagnosis Rates

Source: Shutterstock
International organizations emphasize early diagnosis of cervical cancer, and countries around the world are implementing various measures to increase cervical cancer detection rates by improving accessibility, policies, and technology.
As the role of diagnostic technology becomes increasingly important in improving cervical cancer detection rates and linking diagnosis to treatment, the cervical cancer diagnostic device market is introducing systems that develop and apply innovative technologies aimed at eliminating the disease. In line with this trend, NOUL has developed the miLab™ CER, an automated cervical cytology platform, showcasing an efficient diagnostic system designed to improve the diagnostic environment through innovative technology.
NOUL’s Commitment to Contributing to Cervical Cancer Elimination Through Innovation Technology
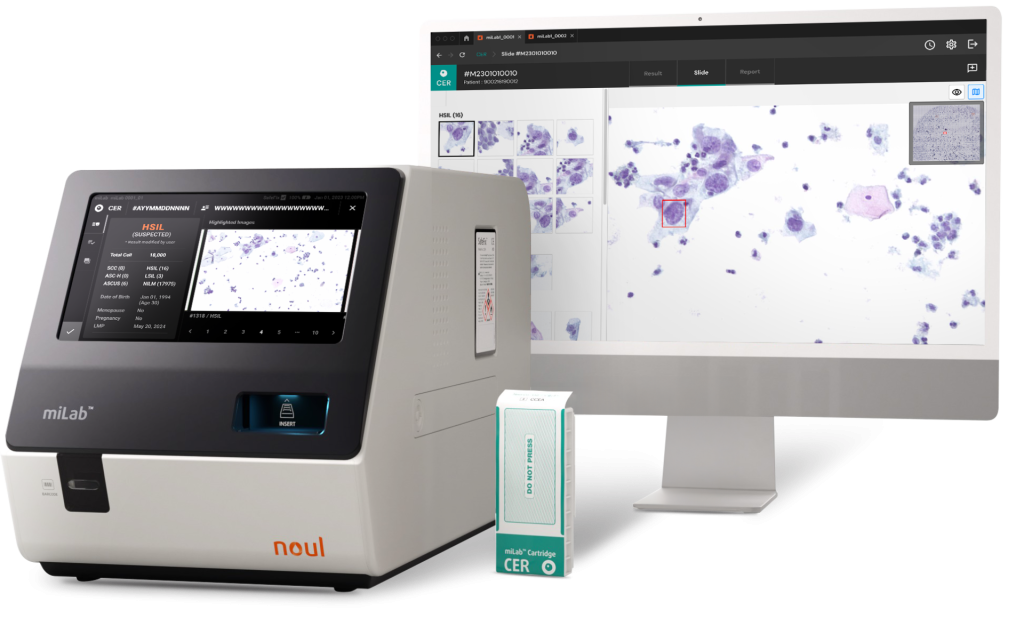
Source: noul
NOUL has developed the automated cervical cytology platform, miLab™ CER. Unlike conventional cytology platforms requiring centralized labs and expert pathologists, miLab™ CER enables high-quality, AI-assisted cervical cancer screening in resource-limited settings—all within a compact, portable device. This system simplifies the complex process by performing staining, washing, and drying of prepared cervical cell slides within a single cartridge. Notably, it reduces the traditionally complex Papanicolaou staining procedure to five steps, significantly improving the existing workflow. The automated testing process also enhances diagnostic efficiency, enabling a higher volume of examinations.
Additionally, our on-device AI analyzes cervical cell images and provides precise and consistent diagnostic results according to the Bethesda System (TBS) classification of cancer stages. This enables specialists to more easily and conveniently assess the progression of cervical cancer. The device is portable and compact, allowing use in limited spaces, and it can replace the functions of a diagnostic laboratory without the need for additional infrastructure investment, making it suitable for use as a point-of-care (POC) device.

Source: Shutterstock
miLab™ CER’s technological innovations drastically reduce the time required for diagnostic testing, enabling same-day completion from examination to diagnosis. As a result, patients with abnormal findings can be promptly connected to treatment or additional testing, while also reducing the time and financial burdens associated with hospital visits. The provision of same-day results enhances the convenience and efficiency of screening, contributing to increased screening rates. Especially in areas with limited healthcare access or inadequate medical infrastructure, this system enables immediate diagnosis, helping to reduce disparities in healthcare services and ensuring that everyone can experience high-quality diagnostic care.
NOUL’s Approach to Addressing Global Health Concern
NOUL’s miLab™ CER was selected as one of the top three recommended products by UNITAID in 2024, recognized for its outstanding performance alongside global diagnostic leaders such as Roche and Hologic. Furthermore, NOUL recently signed agreements to supply miLab™ CER to six Central American countries, including Panama, the Dominican Republic, Costa Rica, and Nicaragua, providing an optimal solution for regions where improvements in cervical cancer screening rates are urgently needed.

Source: noul
As interest in health equity and fairness continues to grow in the United States and Europe, there is an increasing demand for accessible healthcare services. NOUL believes that miLab™ CER can play a key role in addressing these issues and plans to further expand its presence in global markets. In June 2025, NOUL officially launched a new version of miLab™ CER. With its outstanding performance and accessibility, miLab™ CER is set to redefine cervical cancer screening.
To learn more about NOUL’s innovative solution, follow the link below.

