NOUL is proud to announce that the company has obtained a US patent for fluorescence imaging tissue diagnostics. This patent grants NOUL exclusive rights to its fluorescence-based tissue diagnostic technology in the U.S. market. With significant investment in this next-generation technology, we’re confident we can revolutionize diagnostics, focusing on improving accuracy, efficiency, and accessibility.
It’s part of NOUL‘s overall mission. We work globally to secure better healthcare outcomes for patients, developing innovative technologies like the miLab™ platform that helps provide AI-powered testing facilities in resource-deprived regions.
What is NGSI Technology?
 Source: NOUL
Source: NOUL
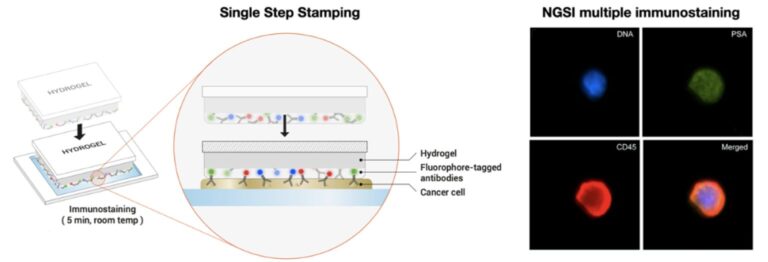
Next Generation Staining and Immunostaining technology, commonly known as NGSI, is a revolutionary advancement in diagnostic testing. This innovative approach utilizes hydrogel-based staining patches infused with staining agents, eliminating the need for conventional liquid reagents. By integrating staining and immunostaining into a solid-phase format, NGSI technology significantly improves efficiency, reduces waste, and enhances precision in diagnostic workflows.
NGSI’s differentiation lies in its hydrogel-based stamping technology, which offers several key advantages:
- Reduced Use of Reagents – NGSI minimizes reagent consumption by up to 99%, making it a cost-effective and sustainable alternative to traditional staining methods.
- Water-Saving Technology – By eliminating washing and drying processes, NGSI prevents wastewater generation, making it an environmentally friendly solution.
- Miniaturization & Process Integration – Unlike conventional staining methods that require pumps, plumbing, and extensive reagent handling, NGSI enables a compact, fully integrated diagnostic process, enhancing lab efficiency and accessibility.
Our fluorescence imaging technology complements NGSI’s solid hydrogel patches, allowing for highly precise staining with minimal error rates. These patches expedite the staining process while supporting multiplex staining options, enabling comprehensive cellular analysis in fields such as oncology, cytology, and histology.
NGSI technology is particularly beneficial for point-of-care diagnostics, research laboratories, and resource-limited settings, where reducing time, cost, and chemical use is crucial. By simplifying workflows and eliminating the reliance on liquid reagents, this groundbreaking approach enhances lab sustainability without compromising staining quality. The potential for cancer diagnostics, immunohistochemistry, and morphological tissue analysis is immense, paving the way for faster, more accurate, and eco-friendly diagnostic solutions.
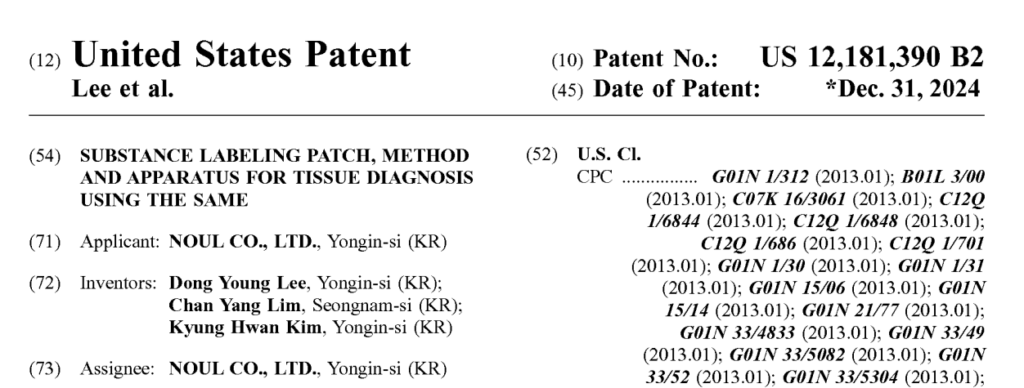
The US Patent: A Game-Changer for Tissue Diagnostics
 Source: USPTO
Source: USPTO
The new US patent covers our fluorescence imaging technology. This advanced diagnostic solution can detect multiple biomarkers simultaneously rather than performing more than one test. Not only does this streamline diagnostics, but the real-time fluorescence gives a better visualization of target substances. The process works by exposing the tissue or cells to specific fluorescent dyes or markers that attach to cellular components or molecules of interest. For example, it could see if breast cancer cells contain estrogen receptors, determining whether estrogen-related medications would be effective.
Moreover, it provides crucial information about the tumor microenvironment. This includes non-cancerous cells, blood vessels, immune cells, signaling molecules, and the extracellular matrix. Advanced diagnostic solutions can use multiple fluorescent markers concurrently to target different aspects of the tumor microenvironment, providing a detailed analysis of what’s going on.
The other benefits? Well, it’s not just about greater accuracy in diagnosis. This form of fluorescence imaging is faster, more precise, and allows earlier detection of diseases, thereby improving patient outcomes. Plus, clinicians can personalize each test to the patients, offering detailed and case-specific insights into tissue samples.
Global Expansion and Clinical Validation
 Source : NOUL
Source : NOUL
NOUL remains committed to expanding our global footprint. Already, we enjoy several collaborations with leading institutions such as Harvard Medical School and Massachusetts General Hospital. Now, we’re taking these partnerships to the next level as we validate the clinical efficacy of NOUL‘s advanced diagnostic solutions, including the miLab™, a US FDA Class 1 medical device.
As a global company, each patent we secure lets us work across multiple jurisdictions, giving patients, doctors, and researchers access to our groundbreaking diagnostics. Recently, we received a similar patent in Brazil as part of our mission not just to supply advanced markets like the US but also to enter and thrive in emergency healthcare markets. Furthermore, securing multiple medical device patents ensures the protection and advancement of our proprietary technologies.
Currently, NOUL is pushing forward in several regions, including Europe, Asia, South America, and now North America. It’s part of our goal to make high-quality diagnostic solutions accessible throughout the world.
FDA Listings and Market Penetration
 Source : Freepik
Source : Freepik
NOUL successfully secured listing for its US FDA Class 1 medical device, the innovative miLab™ platform. It marks a major milestone in the company’s expansion into the US healthcare market. Such listings are critical for access to one of the world’s largest healthcare markets, building trust and credibility among healthcare providers and institutions. Adhering to these stringent regulatory standards helps pave the way for wide adoption of the miLab™, alongside further research into its capabilities.
In addition to the historic FDA listing, we continue to forge strategic partnerships to strengthen our presence in international markets. One notable collaboration is with Limbach Group SE in Europe. Each partnership allows us to penetrate deeper into regional markets, supported by a local player. This market penetration strategy provides a secure platform to scale operations in each key region.
For example, NOUL leverages Limbach Group’s extensive distribution network, customer base, and other capabilities to facilitate a smoother entry into Europe’s healthcare markets. Now, we’re hoping to achieve the same in the US.
Additionally, NOUL continues to expand its medical device patent portfolio, reinforcing our leadership in the development of cutting-edge diagnostic solutions. Our ongoing efforts in securing new medical device patents highlight our commitment to innovation and regulatory excellence.
Pioneering the Future of Diagnostics
 Source : NOUL
Source : NOUL
Securing the US patent for fluorescence imaging isn’t just a landmark moment for NOUL; it represents the next step in cutting-edge tissue diagnostics. With greater precision, faster workflows, and more options for personalized medicine, this novel technology will revolutionize how we think about diagnostics. It’ll allow for early detection of disease and provide greater insights into the tumor microenvironment.
Alongside this development, the miLab™ is now a US FDA Class 1 medical device capable of rapid blood testing by harnessing the power of AI. Each new step reaffirms NOUL’s commitment to advancing the field of diagnostics. Our vision for the future will transform healthcare diagnostics into a more accessible, efficient, and precise system not just for a few advanced economies but for everyone everywhere.
Explore our productions and solutions if you’re ready to learn more about NOUL‘s mission. We provide detailed breakdowns of how they work and who can benefit. Contact our team if you want to partner with NOUL or trial our products in practice. We’re here to help.

