
Source: Google
Compliance has become a defining requirement for AI diagnostics as these technologies move from innovation to real-world clinical adoption.
AI-based diagnostic technologies are reshaping efficiency and accessibility across healthcare systems. As automation and artificial intelligence are introduced into diagnostic fields that traditionally require skilled personnel and significant time, levels of standardization and operational consistency have improved markedly. In settings facing workforce shortages or geographic constraints, these advances translate directly into improved access to essential diagnostic services.
As these technologies become more widely deployed, expectations around reliability and accountability have also increased. Technical performance alone is no longer sufficient to earn trust at a global scale. Because medical devices are directly linked to patient safety, how a technology is developed, manufactured, and managed is increasingly scrutinized alongside its clinical capabilities. In today’s in vitro diagnostics (IVD) market, alignment with international regulatory standards has therefore become a core element of competitiveness.
Diagnostic devices deployed across multiple countries and healthcare systems must demonstrate consistent quality management, safety, and clinical validity under unified criteria. This expectation is especially pronounced in disease areas with high public health impact, where diagnostic accuracy and accessibility are closely tied to national health outcomes. Regulation has thus emerged as a prerequisite for real-world adoption rather than a secondary consideration.
MDSAP and IVDR: Shifting Regulatory Expectations for Global AI Diagnostics

Source: Google
For AI diagnostics to be adopted in routine practice, it is no longer enough to demonstrate strong analytical performance. Manufacturers must also clearly show the quality standards and safety management systems under which their technologies are developed and operated. These expectations are now formalized through global regulatory frameworks, most notably the Medical Device Single Audit Program (MDSAP) and the European Union’s In Vitro Diagnostic Medical Device Regulation (IVDR).
MDSAP is a quality audit program jointly recognized by regulatory authorities in the United States, Canada, Japan, Brazil, and Australia. It evaluates a manufacturer’s quality management system (QMS) through a single audit, reducing the need for repetitive country-specific inspections and enabling more efficient access to multiple markets. In public procurement processes and large hospital networks, MDSAP certification is increasingly treated as an indicator of international-level quality and operational maturity.
In parallel, the EU IVDR replaces the previous IVDD framework with significantly strengthened requirements for IVDs. IVDR expands oversight beyond analytical and clinical performance to include software change control, risk management, and post-market surveillance. For AI-based diagnostics, particular attention is paid to how algorithm updates and software modifications are validated, documented, and controlled in relation to patient safety.
Under current EU law, manufacturers must comply with IVDR requirements to place or maintain IVD products on the European market. IVDR transition is therefore mandatory; failure to comply can result in restrictions on market access and distribution. Because CE marking is referenced by many countries outside the EU, the implications of IVDR extend beyond Europe itself.
Regulatory Readiness and Technical Foundations: The Case of Noul

Source: Google
Rising regulatory expectations require manufacturers to rethink both technical architectures and organizational processes. In AI diagnostics, this extends beyond algorithm performance to automation, reduction of user-dependent variability, and structured software lifecycle control—factors that influence both regulatory compliance and real-world usability.
Within this context, Noul represents an example of a company that has built its technology and quality systems with global regulatory alignment in mind. In November 2025, Noul obtained MDSAP certification, demonstrating that its manufacturing and quality management practices meet internationally recognized standards. This supports regulatory credibility across multiple regions, including the Americas and Asia.
In Europe, Noul has accumulated market experience under the IVDD-based CE framework and is now progressing through the mandatory transition to IVDR. This process reflects a shared challenge faced by all IVD manufacturers seeking to maintain EU market access.
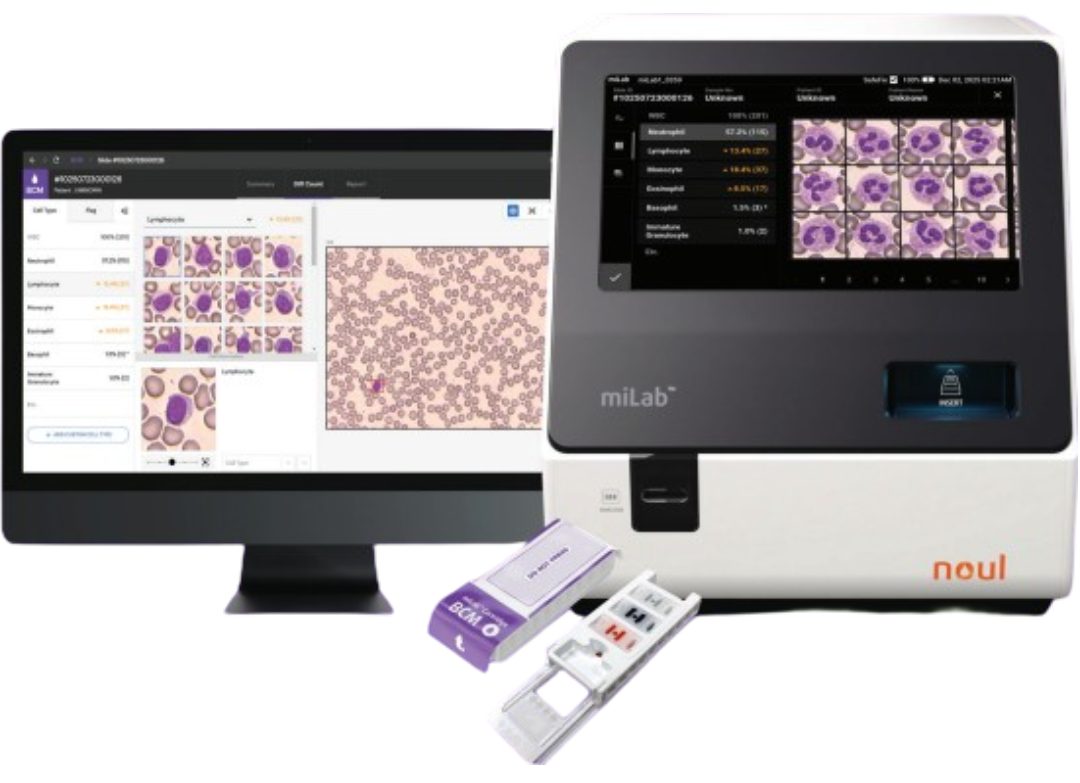
From a technical perspective, Noul’s diagnostic platform, miLab™, is designed to automate the entire workflow from sample preparation and digital imaging to on-device AI analysis. This structure minimizes variability related to user skill or testing environment and supports the standardization and reproducibility expected by regulators. Core technologies protected by multiple patents, combined with years of development experience, provide a technical foundation that supports regulatory review and ongoing compliance.
Translating Certification into Global Market Adoption
Source: noul
Regulatory requirements shape not only documentation but also how diagnostic technologies are designed, deployed, and scaled. These requirements are reflected directly in the architecture of AI diagnostic platforms and their adoption in practice.
miLab™ is an AI-powered point-of-care diagnostic platform applied to disease areas with significant global health burden, including malaria, blood cell analysis and complete blood counts (CBC), and cervical cancer screening. By automating the entire diagnostic process within a single device, the platform aims to deliver consistent diagnostic quality regardless of operator experience or testing environment.
To date, miLab™ has been deployed in more than 28 countries across hospitals, research institutions, and public health programs. This breadth of use indicates that the platform extends beyond pilot projects and controlled settings, enabling repeated use across diverse healthcare contexts.
The platform-based structure also supports expansion into multiple diagnostic areas using the same core device. This flexibility allows healthcare providers to respond to evolving diagnostic needs without replacing existing infrastructure, improving operational efficiency over time. Such characteristics are relevant in both public health programs and laboratory settings where scalability and long-term usability are critical.
Looking Ahead: Building Toward Global Medical Device Leadership

Source: noul
Looking forward, Noul is preparing for a next phase beyond immediate regulatory compliance, with the goal of expanding its presence as a global medical device company. Having accumulated experience with MDSAP and the IVDR transition, the company is positioned to review and prepare for regulatory pathways in additional major markets, including the United States. These efforts reflect a longer-term process aimed at sustained global usability rather than near-term certification outcomes.
miLab™ has evolved with regulatory rigor in mind, incorporating automation and software-centric design principles that support expansion into regions with higher regulatory thresholds. This foundation also enables consideration of new diagnostic applications, geographic markets, and business models as regulatory frameworks continue to mature.
Ultimately, Noul’s growth trajectory is shaped not by a single certification but by the accumulation of regulatory experience, technical maturity, and real-world deployment. How miLab™ expands into new clinical settings and markets will continue to emerge from this preparation—at the intersection of compliance, technology, and global healthcare needs.

