Countries located in Central America exhibit significant disparities in their healthcare environments. For instance, Costa Rica and Panama boast advanced medical infrastructure and service quality, attracting international medical tourists. In contrast, countries like Nicaragua, Honduras, and El Salvador face challenges such as underdeveloped or insufficient public healthcare systems, with particularly limited access to medical services in rural areas.
One of the leading causes of death among women in this region is cervical cancer. Although cervical cancer is a highly treatable disease when detected early, the imbalance between public healthcare services and private medical institutions in Central America prevents many citizens from accessing high-quality medical care. In response, Central American countries are exploring various strategies to address these infrastructure gaps—one of which is the adoption of innovative medical technologies.
How Central America is Addressing the Global Health Concern
Source: Shutterstock
One of the key characteristics of the healthcare environment in Central America is the active support it receives from international organizations such as the UN, WHO, and various NGOs. With this backing, Central American countries have shown a strong interest in global health issues prioritized by the international community. Among these, cervical cancer stands out as a major concern, with the World Health Organization (WHO) outlining three strategic goals for its elimination. In response, many countries in the region are formulating and implementing national health policies centered around this objective.
Three Strategic Goals to Eliminate Cervical Cancer by 2030
- 90% of girls are fully vaccinated against HPV by the age of 15.
- 70% of women undergo high-performance screening by the ages of 35 and 45.
- 90% of women with precancerous lesions or invasive cancer receive appropriate treatment and care.
Central America is one of the regions with the highest incidence and mortality rates of cervical cancer in the world. In particular, Latin America and the Caribbean report more than three times the number of cervical cancer-related deaths compared to North America. This high burden is primarily attributed to low HPV vaccination coverage, limited access to screening services, and underdeveloped treatment infrastructure.
Incidence and Mortality Rates by Major Central American Countries (Based on GLOBOCAN 2020)
In particular, Nicaragua records an incidence rate of 36.2% and a mortality rate of 20.6%, making it one of the countries with the highest cervical cancer rates worldwide. Across Latin American countries, approximately 63% of women have undergone at least one cervical cancer Pap test, but in Nicaragua, only about 35% of women have been tested. This indicates that lower screening rates are associated with higher cervical cancer incidence, highlighting the urgent need to increase access to cervical cancer screening for more women.
Cervical Cancer Screening Status in Central America
Source: Shutterstock
Cervical cancer screening rates in Central America vary by country, but overall, many fall short of the WHO’s recommended target of 70% or higher. To increase screening coverage, these countries have worked to improve access and accuracy by using traditional Pap smears alongside the introduction of HPV DNA testing in some nations.
Root Causes of High Cervical Cancer Rates in Central America: Beyond Screening
So, is Central America’s high cervical cancer incidence caused by insufficient screening? While it’s true that screening is closely linked to cervical cancer rates, tackling cervical cancer in Central America requires a broader, multi-faceted approach to address the root causes.

Source: Rob Crandall / Shutterstock
Many countries in Central America face significant regional disparities in healthcare services. In particular, testing infrastructure is heavily concentrated in urban areas, leaving rural and remote regions with limited medical access. For example, rural and isolated areas often lack sufficient testing facilities and medical personnel, making high-quality diagnostics difficult. The gap in testing accessibility between urban and non-urban areas is wide, leading many women to forgo screenings due to poor hospital access.
In this region, the commonly used Pap test has low sensitivity, requires skilled personnel, and takes considerable time to deliver results, leading to a high dropout rate among patients after testing. Although HPV testing has been introduced in some countries, its adoption is limited due to financial constraints, high costs, and inadequate transportation infrastructure. Such infrastructure shortages and diagnostic delays are also key factors contributing to the high incidence of cervical cancer.
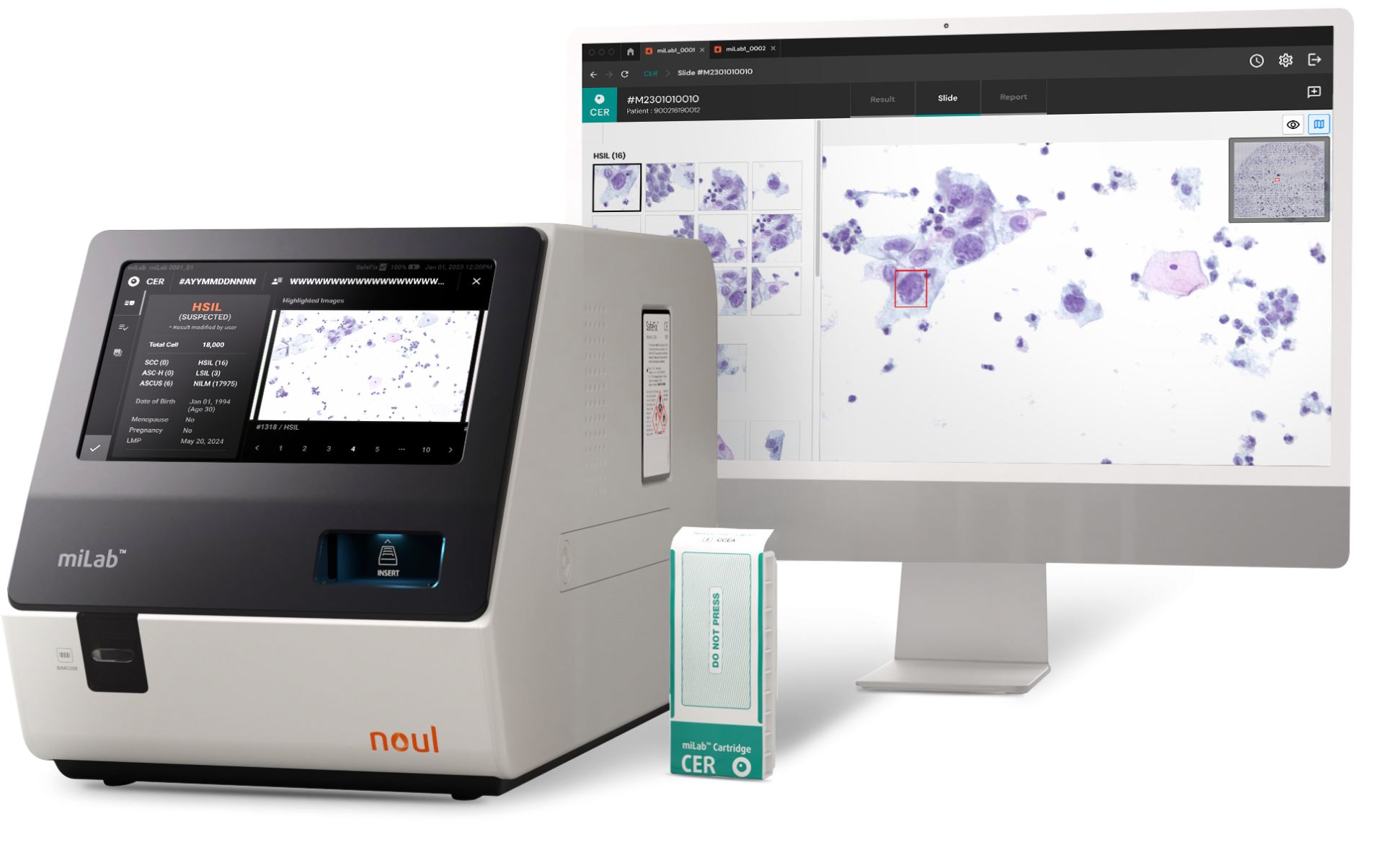
A Cervical Cancer Diagnostic Solution That Enables Same-Day Screening and Treatment: miLab™ CER
Source: noul
To address these challenges and enhance the efficiency of cervical cancer diagnosis, six Central American countries—Panama, the Dominican Republic, Costa Rica, Honduras, El Salvador, and Nicaragua—decided to adopt NOUL’s cervical cancer diagnostic solution, miLab™ CER, in March 2025.
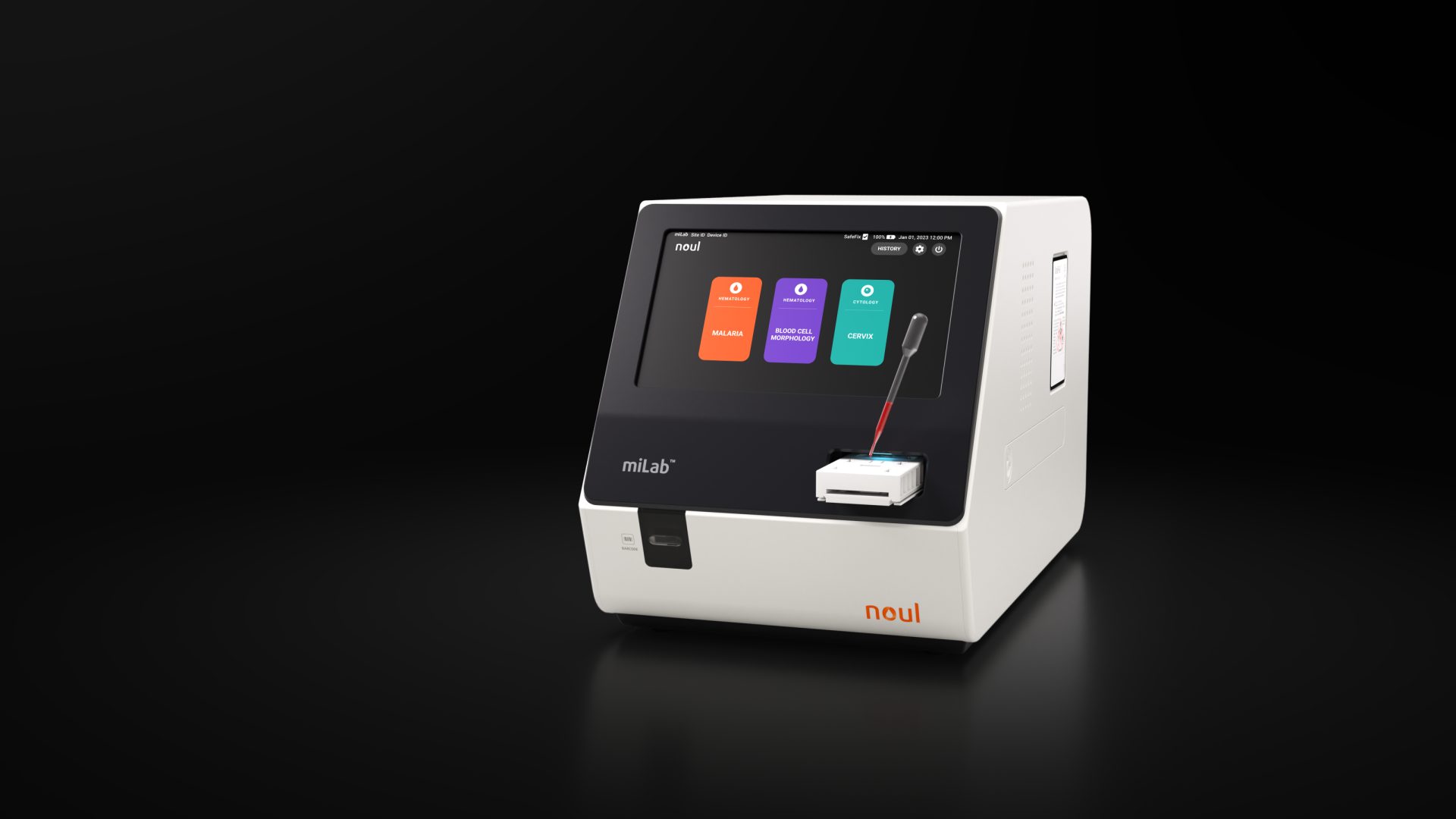
NOUL’s cervical cytology diagnostic system, miLab™ CER, is an advanced solution that simplifies the traditionally complex testing process to maximize efficiency. From staining and washing to drying the prepared cervical cell slides, all steps are performed automatically within a single cartridge. The system handles everything—from slide preparation and image capture to AI-powered interpretation—on one fully integrated device. Thanks to this complete automation, testing efficiency is significantly improved, enabling results to be confirmed on-site in about an hour. This allows not only same-day diagnosis but also immediate review of treatment strategies.

Source: noul
One of the key strengths of miLab™ CER lies in its use of NOUL’s on-device AI, which analyzes cervical cell images and classifies cancer progression with precision and consistency based on the Bethesda System (TBS) criteria. This enables medical professionals to more easily and accurately assess the stage of cervical cancer, offering a streamlined and user-friendly diagnostic experience.
miLab™ CER: A Solution for Cervical Cancer, Emerging as a Global Health Challenge
Source: noul
NOUL’s miLab™ CER recently demonstrated its outstanding performance by presenting research findings at ICC 2025, a prestigious conference in the field of cytopathology. Additionally, it was selected as one of the Top 3 recommended products in the 2024 WHO UNITAID report, alongside global diagnostic leaders Roche and Hologic. Recognized by various international organizations, miLab™ CER is gaining attention as a key solution in cervical cancer diagnosis.
Cervical cancer is a global concern—what solutions can miLab™ CER offer? Learn how miLab™ CER delivers same-day cervical cancer diagnosis to underserved clinics, click the link now to learn more!

