The Point-of-Care (POC) diagnostics market is experiencing rapid growth. As healthcare services expand beyond hospitals to primary care settings, communities, and even home environments, the demand for diagnostic technologies that enable immediate testing on-site is increasing significantly. Technological advancements have further enhanced the quality and capabilities of POC diagnostic systems, allowing for faster and more accurate test results. This, in turn, is a key driver accelerating the growth of the market.
The European Point-of-Care (POC) diagnostics market is projected to grow from approximately USD 8.39 billion in 2024 to around USD 19.34 billion by 2033. Currently, POC diagnostics are widely utilized across various medical areas in Europe, including infectious disease testing, chronic disease management, and emergency and critical care. Among these applications, blood testing accounts for a significant share of the market.
Core of Europe’s POC Diagnostics Market: Blood Testing

Source: Mareks Perkons / Shutterstock.com
In recent years, growing interest in personalized healthcare across Europe has led to a steady increase in demand for blood testing. Many of the most common and essential POC tests—such as those for blood glucose, cardiovascular and metabolic conditions, and blood coagulation—are primarily blood-based. As a result, blood testing holds a dominant position within the European POC diagnostics market.
Major countries such as Germany, France, and the United Kingdom regard blood testing not merely as a diagnostic tool, but as a strategic means to efficiently manage public health and ensure the sustainability of their healthcare systems. Accordingly, public healthcare systems like GKV in Germany, CNAM in France, and the NHS in the UK provide basic blood tests covered by insurance or public funding. These efforts are supported by national-level policy initiatives.
Impact of High-Accuracy Test Results on the Point-of-Care Diagnostics Market
Source: Shutterstock
Although POC diagnostic settings often lack the infrastructure of centralized laboratories and involve simplified procedures, delivering accurate results remains essential. This is because blood tests play a critical role in guiding clinical decisions and have a direct impact on patient safety.
Here are more specific reasons why accurate diagnostics are essential in POC settings:
- Immediate Clinical Decision-Making: Rapid test results at the point of care allow healthcare providers to quickly assess a patient’s condition and make timely clinical decisions.
- Building Trust Between Patients and Providers: Accurate and prompt results help establish trust between patients and medical staff, facilitating smoother consultations and enhancing the overall convenience of POC testing.
- Improved Efficiency in Medical Resource Utilization: Accurate diagnostics enable clear assessment of the urgency and type of care needed, allowing for more efficient allocation of medical resources.
Recently, blood testing in POC settings has been rapidly advancing in performance, driven by ongoing technological innovation. In particular, CBC and blood morphology tests—both essential for early disease screening and ongoing monitoring—are now being accurately implemented in POC environments. This capability is significantly transforming traditional diagnostic workflows.
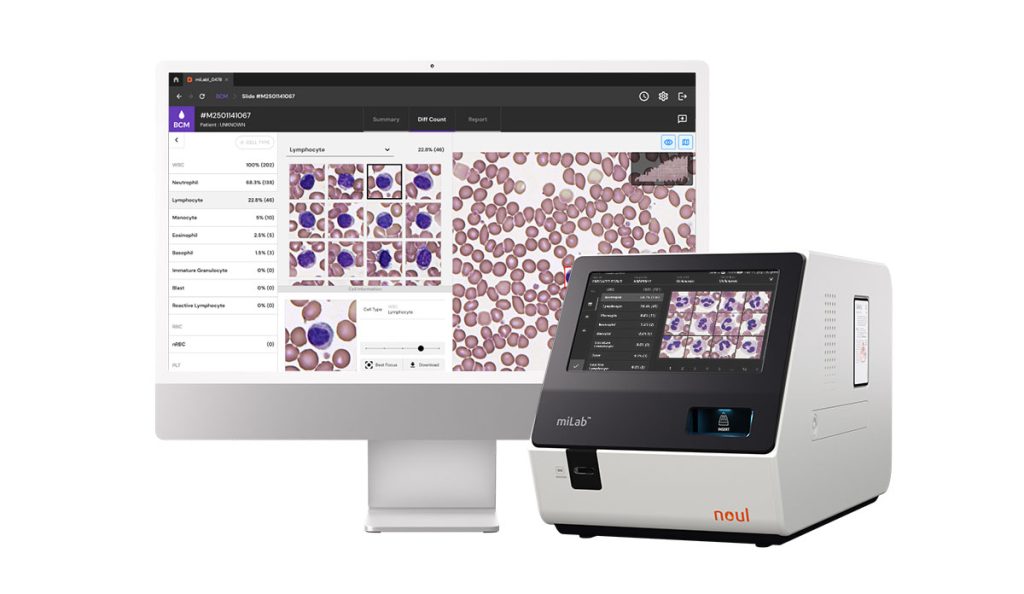
AI-Powered Diagnostic Transforming the Paradigm of Point-of-Care Testing: miLab™ BCM
Source: noul
NOUL’s miLab™ BCM is a leading blood analysis platform that is redefining the paradigm of POC diagnostics through innovative technology. It streamlines the testing process while maintaining high performance, delivering fast and accurate results. Its compact design ensures excellent portability, and the use of disposable cartridges makes it optimized for the POC diagnostic environment in Europe.
miLab™ BCM enables precise diagnostics by integrating Complete Blood Count (CBC) and Blood Morphology analysis within a single device, greatly enhancing user convenience. It automates the entire process—from sample preparation and staining to high-resolution imaging and AI-driven analysis—delivering fast and consistent test results.

Source: noul
Notably, NOUL’s proprietary NGSI(Next Generation Staining and Immunostaining) technology departs from traditional liquid-based methods by using solid-based staining and immunostaining. This innovation dramatically simplifies the complex sample preparation steps—such as staining, washing, and drying—by integrating them into a single disposable cartridge. Additionally, on-device AI accurately and consistently analyzes key CBC parameters and cell morphology, offering an innovative solution that enhances testing efficiency while maintaining diagnostic accuracy.
By automating and integrating the testing process in this way, the system proves highly practical and effective for real-world point-of-care use.
NOUL Participates in EUROMEDLAB 2025, Europe’s Largest Diagnostic Medicine Exhibition

Source: noul
NOUL has recognized the rapid growth in demand for POC diagnostics within decentralized healthcare environments across Europe and the global medical market. To accelerate its entry into the European market, NOUL participated in EuroMedLab, Europe’s largest diagnostic medicine exhibition.
EuroMedLab is the largest conference and exhibition in Europe for clinical and diagnostic laboratory medicine, jointly organized by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). The event attracts over 5,000 professionals from more than 100 countries worldwide. Held from May 18 to 22 in Brussels, this year’s event features participation from numerous global companies, including Roche, Beckman Coulter, and Sysmex.
NOUL set up a booth at this year’s EuroMedLab, providing visitors with the opportunity to experience miLab™’s innovative technology firsthand. Through interactions with professionals from various organizations and corporates, NOUL gained valuable insights to support the development of even better products.
Countries across Europe—including Germany, the UK, the Czech Republic, and Romania—as well as nations in the Middle East and Central Asia such as Kazakhstan and Uzbekistan, showed strong interest in miLab™’s diagnostic automation technology and on-device AI.
Visitors expressed keen interest in all of NOUL’s product lineup, including miLab™ MAL, BCM, and CER. The overwhelming response was evident, with all brochures distributed and a steady stream of visitors stopping by the booth to experience miLab™ firsthand.

Source: noul
During the exhibition, interactions with a wide range of global healthcare professionals visiting NOUL’s booth confirmed the commercialization potential and technological competitiveness of the miLab™ BCM in the European market. The platform’s integration of CBC and Blood Morphology functions is expected to attract significant interest in Europe, where demand is growing for multi-panel tests capable of diagnosing multiple diseases simultaneously.
Additionally, NOUL is actively creating various business opportunities across Europe. With local Business Development Managers based in Germany, France, and the UK, the company closely monitors country-specific regulations, healthcare systems, and diagnostic demands in real time to develop and execute tailored business strategies.
They have identified the European POC diagnostics industry’s continuous demand for improved testing equipment performance to enable more accurate and rapid diagnostics. They are actively showcasing miLab™ BCM at various European exhibitions and conferences, while building collaborative opportunities with large laboratories and hospitals. Notably, in 2024, they achieved a milestone by signing a supply agreement with Limbach Gruppe, Germany’s largest laboratory chain.
NOUL Expands Its Presence in the European Market Based on Innovative Blood Testing Technology

Source: noul
miLab™ BCM, having secured CE-IVD certification as a foundation for its entry into the European market, is currently conducting local clinical trials at leading research institutes and diagnostic laboratories in major hospitals across Germany and France to establish evidence for product commercialization. Additionally, by supplying miLab™ solutions to select Eastern European countries—where testing infrastructure and healthcare access are relatively limited compared to Western Europe—NOUL aims to help reduce healthcare disparities, further strengthening its position within the European market.
Moving forward, NOUL will continue to expand its distribution network and build local clinical case studies, leveraging its technological expertise and reliability to demonstrate the performance and efficiency of its products, ultimately establishing itself as a leading blood testing device provider in Europe.
If you want to learn more about NOUL’s integrated blood testing platform, miLab™ BCM, which is set to create a new paradigm in the European blood testing market, you can find more information through the link.

