Donating blood to others is a life-saving act. When a person finds themselves critically injured, donated blood can ensure that a harmful injury doesn’t become deadly. It helps with everything from surgeries and trauma care to managing chronic diseases like anemia and cancer.
The problem? Well, in certain countries, there is a substantial risk of injecting malaria-contaminated blood. Thorough blood donation testing is essential to prevent transfusion-transmitted infections (TTIs), including malaria.
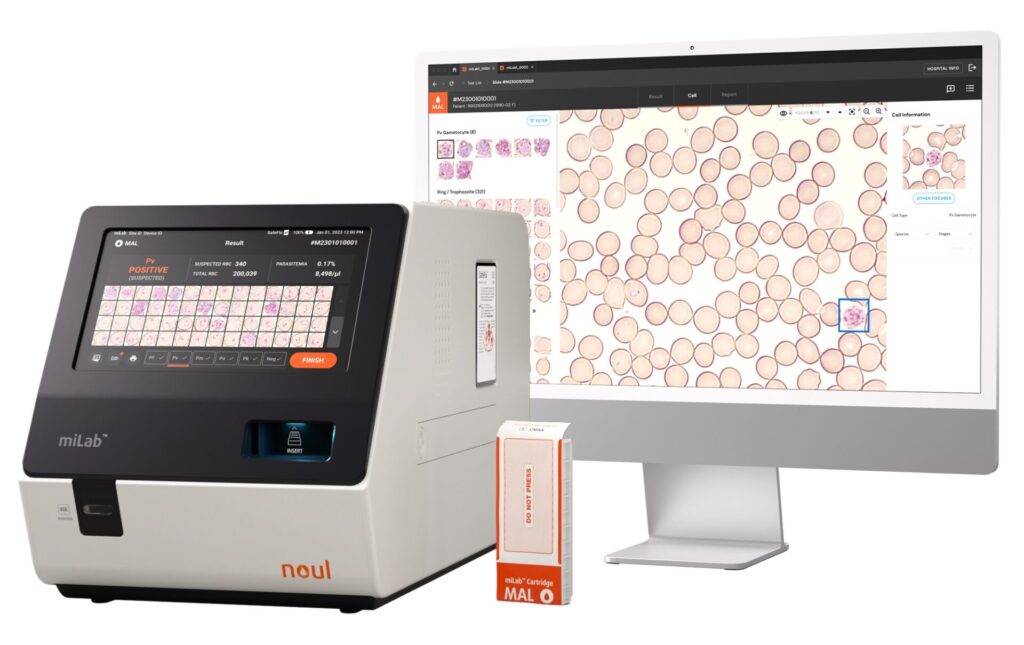
Each blood sample must be tested for malaria, complete blood count (CBC), and other essential factors to ensure safety. NOUL‘s miLab™ is a ground-breaking technology that analyzes a blood sample in a matter of minutes, screening for malaria and other risk factors. It’s set to revolutionize donor blood processing.
Comprehensive Blood Donation Testing
 Source: Freepik
Source: Freepik
Blood donation testing is split into two categories: pre-donation screening and blood testing.
Pre-donation screening evaluates each donor, ensuring they meet the blood donation requirements. That includes performing physical health checks, asking for a travel history, and determining if they’re at risk of malaria transmission. This step minimizes the chance of infection for recipients, ensuring only eligible donors participate.
Blood tests are then administered to the donor’s sample. These tests include:
- Complete blood count (CBC) measures the levels of important blood cells, including red cells, white cells, hemoglobin, and platelets, to ensure their suitability for transfusion.
- Infection disease screening doesn’t just cover malaria. Alongside malaria tests, the blood sample is screened for other TTIs, including HIV and hepatitis.
Performing these tests takes time – adding to the expense and difficulty of providing a steady supply of donor blood. NOUL‘s miLab™, in contrast, performs these analyses within a few minutes, increasing accuracy and reliability compared to human-led testing.
The Role of Malaria Testing in Blood Donation
 Source : Unsplash
Source : Unsplash
Malaria is a life-threatening condition caused by the Plasmodium parasite. It is transmissible via blood transfusion. Conducting a malaria test for each donor is the only way to guarantee that clean, safe blood is administered to patients. With increasing global travel, the risk of malaria is present even in non-endemic regions.
Traditional malaria tests involve microscopy, using a blood smear technique to laboriously inspect the sample for malaria. This takes time and significant expertise. Automated diagnostic solutions offer a faster alternative, as platforms like the miLab™ automate the process using AI. This allows for malaria tests and other blood donation requirements to be more readily integrated into the screening process, ensuring everyone receives pathogen-free blood. This isn’t optional; it’s essential.
Donor Blood Processing: From Collection to Storage
 Source : Flickr
Source : Flickr
Donor blood processing follows stringent regulations and rules to safeguard against harm. It is collected using safe methods, such as accurate labelling and correct technique, to maintain traceability and integrity.
The blood sample then undergoes essential tests, such as CBC and malaria screening. Afterwards, the sample is separated into its component parts: red blood cells, plasma, and platelets, which are administered for specific medical purposes. These blood components are stored under controlled conditions to preserve their quality and can be distributed to specific facilities on demand.
Currently, one of the biggest bottlenecks in this process is laboratory testing. Automated systems, like the miLab™, speed up this process by optimizing donor blood processing. The platform minimizes errors and ensures that blood goes from donor to recipient quickly without risking any lives.
Importance of Phlebotomy in Blood Donation Testing
 Source : Flickr
Source : Flickr
What is phlebotomy? Phlebotomy is the process of collecting a blood sample (literally coming from the Greek words “phlebo-,” meaning “vein” and “-tomy,” meaning “cutting”). Usually, it is carried out by a nurse, doctor, or specialist phlebotomist. Whoever is collecting the blood must follow an order of draw to prevent contamination if multiple tubes are required – each tube undergoes a specific test.
This draw order applies to blood donation testing. It is crucial that CBC and malaria tests aren’t confused or performed incorrectly as a result of errors in the collection of blood or labelling of tubes. Such cross-contamination or error could compromise results. Thankfully, most phlebotomists are highly trained to adhere to blood donation requirements, ensuring reliable outcomes.
Global Blood Safety Standards and Malaria Testing
 Source: NOUL
Source: NOUL
Blood donation testing isn’t simply advisable; it’s part of the World Health Organization’s (WHO) guidelines to prevent TTIs. Malaria screening is an essential step in ensuring blood safety. Under universal blood safety protocols, patients are protected from blood-transmitted diseases in both endemic and non-endemic regions.
With increasing global travel, formerly safe, “non-endemic” regions are now facing rising numbers of malaria cases. Take the European Union (EU), for example. In 2023, there were around 7,200 cases of malaria in the EU – an increase of over a thousand cases year-on-year. Italy recently saw its first native case of malaria since the 1960s.
With shifting global trends, malaria screening has become essential even in non-endemic regions. It has to become a core part of comprehensive donor blood processing.
The obvious solution is NOUL’s miLab™ MAL – a system specifically designed to rapidly test blood samples for malaria. Harnessing the power of AI, it can fit seamlessly into existing workflows, providing a safe and efficient testing platform. In fact, we believe it can help blood banks meet and even exceed global safety standards.
Disclaimer: Blood collection and transfusion procedures are subject to strict regulatory oversight due to their direct impact on patient safety. Blood collection devices often require higher medical device classifications, and errors in blood typing can lead to life-threatening transfusion reactions. miLab™ MAL is designed for malaria screening and does not replace mandatory blood compatibility testing.
Conclusion
The importance of rigorous blood donation testing cannot be overstated, as it ensures that all blood donations are safe and free from pathogens, including malaria.
NOUL‘s miLab™ MAL revolutionizes this process by automating malaria testing, delivering unmatched accuracy and speed, which is vital, especially in resource-limited settings. This technology not only simplifies blood donation testing but also aligns with global safety standards, enhancing the overall safety and efficiency of blood transfusions.
Partner with NOUL to leverage the capabilities of miLab™ MAL, ensuring a safer blood supply for patients. Contact us today to advance your diagnostic capabilities.

