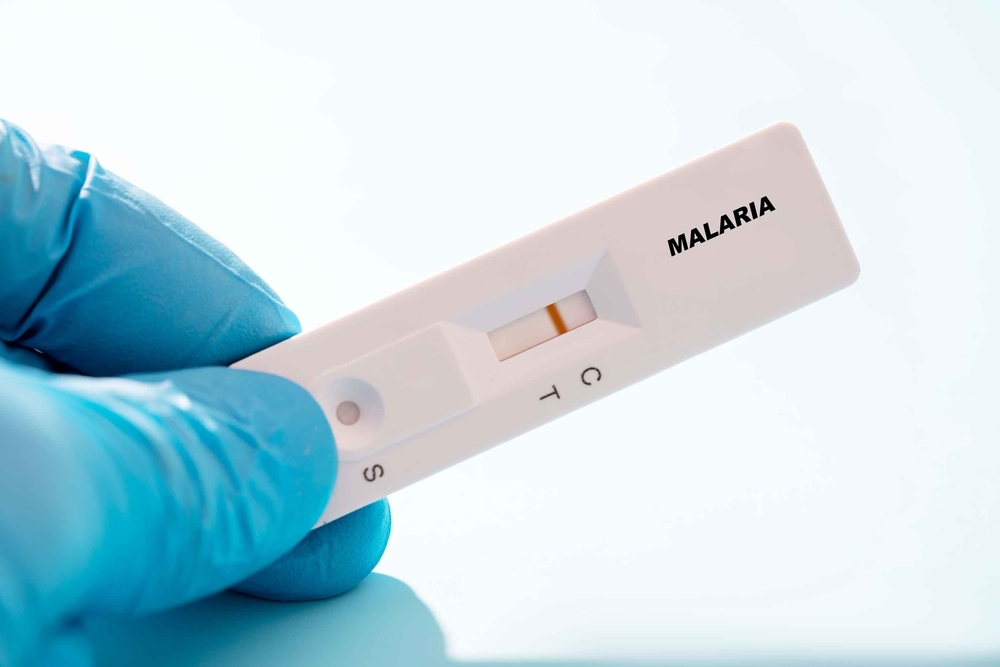
RDT stands for Rapid Diagnostic Test, a widely used method for the quick detection of diseases, including malaria. In malaria diagnosis, a single drop of blood can reveal the presence of infection within minutes, making RDTs an essential tool in many regions. In malaria-endemic countries, more than 70–80% of all diagnoses rely on RDTs. In point-of-care (POC) settings, over 90% of testing is estimated to depend on RDTs, underscoring their role as the primary diagnostic method globally.
Source: Shutterstock
Despite their widespread use, RDTs have certain limitations. False-negative or false-positive results can occur, particularly in cases of low parasite density or when the hrp2/3 genes are deleted. Additionally, RDTs are highly sensitive to storage and usage conditions, with performance often deteriorating in hot and humid environments. Although other diagnostic methods—such as microscopy and molecular testing—are also used for malaria detection, they require well-established infrastructure and trained personnel. As a result, their application remains limited in many malaria-endemic regions where resources are scarce.
Despite the risk of false-negative and false-positive results, RDTs remain the most widely used method for malaria diagnosis worldwide. This is largely due to their low cost and ability to deliver results within minutes—critical advantages in resource-limited settings. In fact, in 2023, the World Health Organization (WHO) reported a record-high global supply of 448.5 million RDTs. In sub-Saharan Africa, RDTs are used as the primary diagnostic tool in nearly all first-line healthcare facilities, reinforcing their dominant role in frontline detection.
How Diagnostic Errors in Malaria Testing Delay Treatment and Increase Risk
Source: Shutterstock
In 2024, the World Health Organization (WHO) received reports from multiple countries of confirmed malaria patients showing only faint positive lines on RDTs. In the past, such faint lines were often interpreted as negative results. However, repeated cases involving patients with low parasite density still producing faint positive lines have drawn attention to this issue. As a result, misdiagnosis and treatment delays have also been reported in connection with these ambiguous test outcomes.
A recent study conducted at Iganga Hospital in Uganda found that among 225 febrile patients who tested positive for malaria using the RightSign Malaria Ag HRP-II/Pan Plasmodium RDT, 48% were found to be negative by microscopy. Of those, 45% were also confirmed negative by LAMP testing, indicating an approximate false-positive rate of 20%. Additionally, the BinaxNOW® RDT has shown false-positive results triggered by immune responses such as the presence of rheumatoid factor (RF), with a reported false-positive rate of about 13%.
Source: Shutterstock
These false-negative and false-positive diagnoses have serious implications not only for individual patient care but also for public health. In March 2025, the World Health Organization (WHO) issued an official information notice to national regulatory authorities and malaria program managers worldwide. The notice aimed to break the cycle of misdiagnosis, treatment delays, and the resulting increases in malaria-related morbidity and mortality. Specifically, it advised that faint positive lines on RDTs should not be interpreted as negative, and emphasized the importance of appropriate follow-up actions to avoid delays in treatment.
WHO’s Information Notice for Accurate Malaria Testing

Source: RH / Shutterstock
- In response to numerous reports of faint test lines in confirmed malaria cases being misinterpreted as negative results, WHO has issued a formal recommendation that even faint lines on RDTs should be considered positive.
- To address this issue and prevent further misdiagnosis and delays in treatment, WHO has requested the following actions from all national regulatory authorities and malaria control programs:
- Strengthen training and retraining for RDT users, including healthcare providers and laboratory personnel.
- Strictly monitor storage and transportation conditions to ensure they comply with the manufacturer’s specifications.
- Establish systems to compensate for visual inspection limitations, such as poor eyesight among testers.
- Promptly report abnormal or questionable results to manufacturers and relevant authorities.
- In cases where product quality is in doubt, consider follow-up performance evaluations or recalls.
- WHO has officially urged all national health authorities and manufacturers to disseminate this notice widely, as part of global efforts to protect public health and maintain trust in RDT reliability.
This official notice from WHO indicates that the issue of false-negative and false-positive malaria diagnoses is not limited to a single country, but rather reflects a global trend. WHO typically issues such guidance only when patterns have been consistently observed across multiple regions—signaling a real and growing concern worldwide. This development may mark the beginning of a paradigm shift in malaria diagnostics. It also underscores the need for stronger quality standards for IVDs and could accelerate the adoption of digital diagnostic devices in malaria-endemic regions.
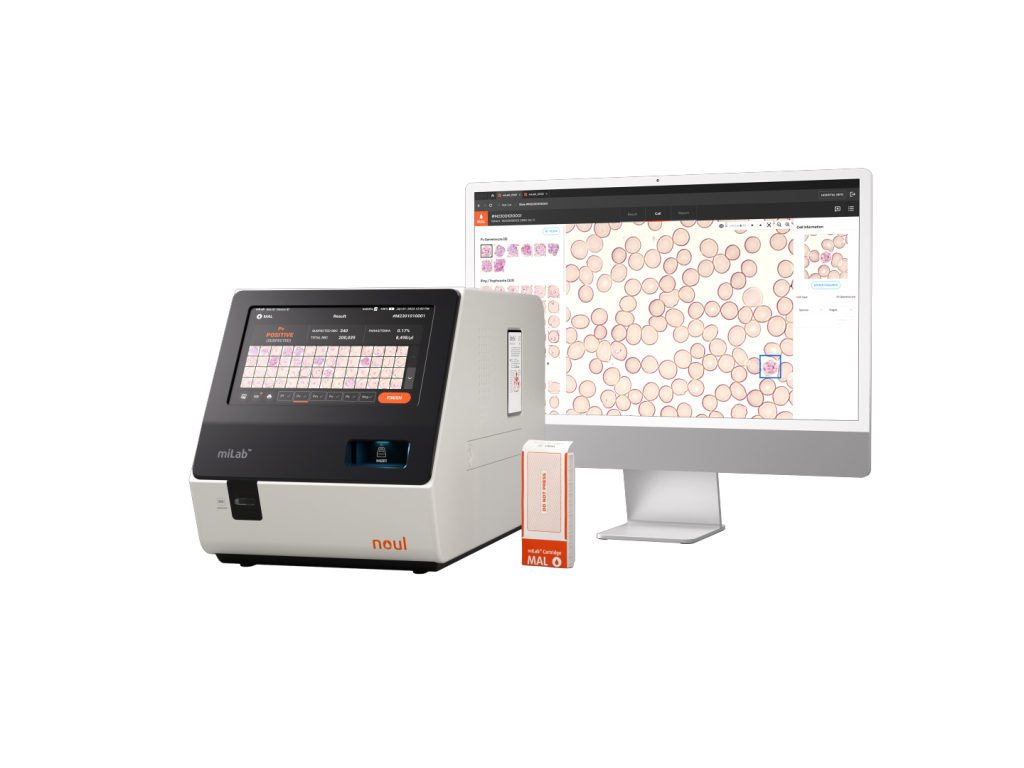
miLab™ Solution Optimized for Malaria Diagnosis with Excellent Performance to Prevent Misdiagnosis
Source: noul
miLab™ MAL was introduced as an innovative product entering the Scale-up stage at UNITAID 2022. In one clinical evaluation, it demonstrated outstanding performance by achieving 100% sensitivity and specificity, proving its high diagnostic accuracy.
This digital microscope-based solution automates the testing process while using the same diagnostic principles as traditional microscopy, enabling greater efficiency. NOUL’s solid-staining technology, NGSI(Next Generation Staining and Immunostaining), integrates the entire microscopic diagnostic procedure, and on-device AI analyzes the results to deliver precise and consistent outcomes.
Unlike RDTs, the platform employs a morphological diagnostic method, allowing accurate testing regardless of pfhrp2/3 gene deletions. This approach significantly reduces false negatives and false positives, ensuring precise diagnosis and facilitating effective treatment linkage.
Can miLab™ MAL Replace RDTs?

Source: noul
miLab™ MAL has strong potential to overcome the limitations of Rapid Diagnostic Tests (RDTs) and establish a new paradigm in malaria diagnosis. First, while RDTs often miss Plasmodium falciparum with hrp2/3 gene deletions or produce false negatives and positives due to faint reaction line interpretation, miLab™ MAL uses a morphological diagnostic method rather than an antigen-based approach, effectively addressing these genetic and visual limitations.
Moreover, its AI-powered automated reading system delivers precise and consistent results without the need for skilled microscopy technicians. By automating the entire process—from sample preparation to reading—miLab™ MAL reduces patient diagnosis time to around 10 minutes, significantly improving speed and efficiency compared to traditional PCR or microscopy methods. Additionally, its benchtop portable design automates microscopic examination with just one cartridge, making it highly suitable for point-of-care (POC) environments, even without advanced infrastructure.
Recently, NOUL signed a contract to supply miLab™ MAL to the public health system in Benin, Africa. By enhancing diagnostic efficiency through automation and reliably detecting infections with pfhrp2/3 gene deletions, miLab™ MAL strengthens diagnostic accuracy and quality. This contributes to laying the foundation for reducing malaria incidence and mortality rates in Benin.
NOUL’s Challenge Toward Achieving Global Zero Malaria
Source: flickr
NOUL’s miLab™ MAL aims to contribute to global malaria elimination by enabling accurate diagnosis and supporting more effective treatment linkage. The solution is already being adopted as part of government-led healthcare initiatives, including a public procurement contract with Benin and supply for Kuwait’s national screening program. Most recently, NOUL conducted a field visit across six African countries to identify regions in need of accurate malaria diagnostics and successfully completed product deliveries to support their efforts.
miLab™ MAL is already in regular use in high-burden West African countries such as Benin and Côte d’Ivoire, where its growing demand continues to validate its usability and impact. Beyond Africa and the Middle East, NOUL is actively pursuing regulatory certifications to expand the reach of its innovative miLab™ platform to more countries around the world, with the goal of improving healthcare systems through cutting-edge diagnostic technology.
Explore the innovation behind miLab™ MAL and see how it’s reshaping the future of malaria diagnosis through accuracy, automation, and accessibility.

