
Source: Shutterstock
Among the leading global health challenges, cervical cancer is primarily caused by high-risk HPV infections, accounting for 99% of cases. HPV can be prevented through vaccination, and infections can also be detected early through testing. This makes cervical cancer one of the few cancers that can be eliminated, as it can be identified in its early stages and has a long precancerous phase that spans several years before progressing to cancer.
Through regular screenings such as Pap smears and HPV tests, cervical abnormalities can be detected early, and initiating treatment at this stage can prevent the progression to cancer almost entirely. In addition, highly effective HPV vaccines, advanced screening methods, and early treatment options provide effective ways to eliminate precancerous lesions. Nevertheless, cervical cancer still ranks as the fourth most common cancer among women worldwide, underscoring its status as a persistent and unresolved global public health issue.
WHO’s Cervical Cancer Elimination Initiative

Source: Flicker
As cervical cancer has emerged as a major global public health concern, the World Health Organization (WHO) has established strategies and launched a range of programs aimed at its elimination. WHO’s goal is to reduce the incidence of cervical cancer to fewer than 4 cases per 100,000 women, while the current global average incidence stands at 14.12 cases per 100,000.
WHO has set the 90-70-90 strategy as a target to be achieved by 2030 and is currently driving initiatives to realize it. This strategy outlines three key goals: achieving 90% full HPV vaccination among girls by age 15, ensuring that 70% of women are screened with a high-performance test at least once by ages 35 and 45, and providing treatment to 90% of women diagnosed with precancerous lesions or invasive cervical cancer.
In 2018, under the leadership of the WHO Director-General, a global call to action against cervical cancer was officially launched. Following the adoption of the “90-70-90” strategy in 2020, WHO has been working in collaboration with various organizations, including UNICEF and IAG, to establish accountability and governance structures to drive the strategy forward. Notably, in partnership with the International Agency for Research on Cancer (IARC), WHO was the first to scientifically establish the link between HPV and cervical cancer, while also conducting evaluations on the safety and efficacy of HPV vaccines. Today, this collaboration continues to provide the scientific evidence and policy recommendations necessary to advance WHO’s cervical cancer elimination initiative.
Ongoing Programs for Cervical Cancer Elimination

Source: WHO
WHO’s Cervical Cancer Elimination Initiative is advancing the “90-70-90” strategy through a range of programs, global events such as the Global Cervical Elimination Forum and World Cervical Cancer Elimination Day, as well as collaborative projects with international partners.
In addition, WHO and its partners conduct online training sessions, workshops, and educational programs for healthcare professionals, policymakers, and program managers. Expert working groups are established in specific areas such as HPV, screening, and precancer and cancer management to develop guidelines and review the latest evidence. Furthermore, through ongoing collaboration with organizations such as IARC, FIGO, and ASCO, WHO continuously updates clinical guidelines and policy recommendations, providing evidence-based support to accelerate the implementation of effective cervical cancer elimination strategies.
Cervical Cancer Diagnostic Methods
Source: Shutterstock
As these various programs work to advance cervical cancer elimination, it is equally important in practice to detect cervical cancer early through accurate diagnosis and ensure linkage to treatment. For precise staging, cervical cancer diagnosis is conducted in three sequential steps: screening, diagnostic confirmation, and staging tests.
- Screening Tests: Conducted to detect abnormalities early in asymptomatic individuals.
- Examples: Pap smear, Liquid-Based Cytology (LBC), HPV DNA testing
- Confirmatory Tests: Performed when screening tests indicate abnormalities.
- Examples: Colposcopy, tissue biopsy
- Staging Test: Conducted after a cervical cancer diagnosis to assess the extent of disease progression.
- Examples: MRI, CT, PET-CT, X-ray
Although these systematic and sophisticated approaches have improved diagnostic accuracy, global detection rates remain low due to limited access to screening, lack of awareness, and the time and cost burdens associated with testing. In particular, delays between screening and diagnosis often prevent timely linkage to treatment, posing a significant challenge to cervical cancer elimination.
NOUL’s miLab™ CER
Source: noul
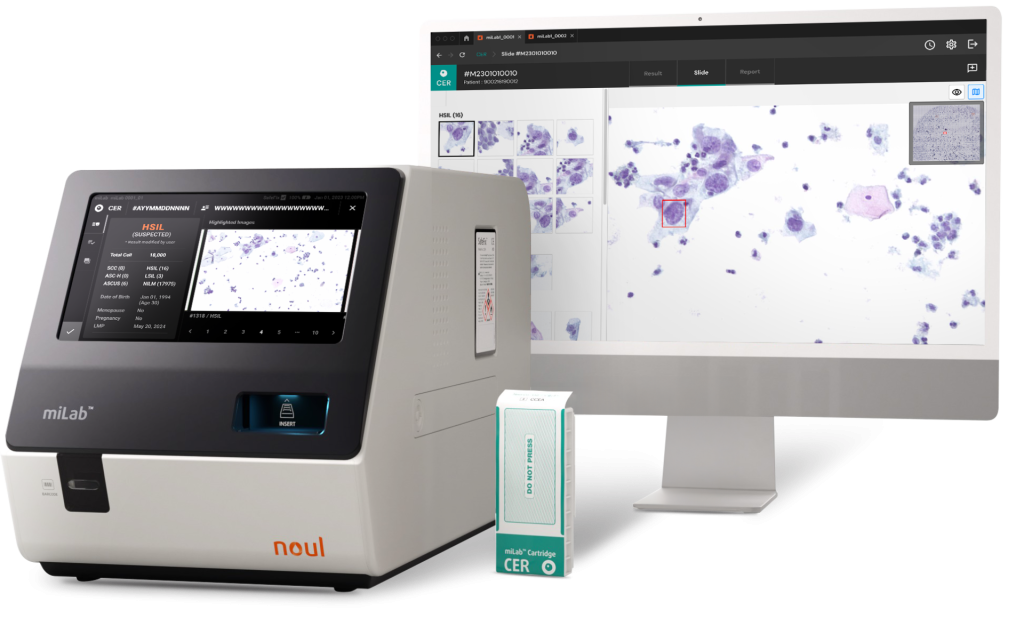
NOUL’s miLab™ CER is a digital microscope designed for analyzing cervical cancer cells and supporting Liquid-Based Cytology (LBC) screening. While LBC screening offers high accuracy, it can be time-consuming, which sometimes prevents seamless linkage from diagnosis to treatment and can contribute to workforce shortages. Recognizing these challenges, Noul has automated the cell analysis process to dramatically improve diagnostic efficiency and established a workflow that enables both screening and diagnosis on a single platform.
The LBC slide containing collected cells is inserted into a cartridge, which is then placed into the device. The system carries out staining, washing, and drying, and provides analysis and result reporting all within the same device. Notably, the on-device AI delivers precise and consistent analyses according to the Bethesda System (TBS) diagnostic categories, providing stage-specific results even in low-resource settings. Additionally, the compact miLab™ diagnostic platform is well-suited for point-of-care (POC) environments, enhancing access to quality healthcare.

Source: unitaid
Notably, in 2024, this product was recognized for its outstanding performance, being selected as one of the Top 3 recommended products for cervical cancer diagnosis in the UNITAID report alongside global diagnostic brands such as Roche and Hologic. Recently, it was introduced to the Latin American region, where HPV vaccine adoption has been rapidly expanding. As a result, in March, supply agreements were signed with six Central American countries—including Panama, the Dominican Republic, and Nicaragua—marking tangible progress in the fight against cervical cancer.
miLab™ CER enhances diagnostic efficiency through a streamlined workflow, providing not only cell analysis but also test results, enabling screening and diagnosis on a single platform. This establishes a continuous solution that bridges the gap from diagnosis to treatment. Leveraging these strengths, the platform aims to expand into global diagnostic markets, contribute to WHO’s cervical cancer elimination strategy, and help achieve global health equity by significantly improving access to high-quality diagnostics.
Learn more about how the miLab™ CER we introduced today is transforming the workflow of cervical cancer diagnosis.

