NOUL is a pioneer in AI-driven healthcare innovation, developing medical diagnostic tools that revolutionize clinical pathology and healthcare accessibility. Little wonder, then, that we showcased our leading technologies at MEDLAB Middle East 2025.
Held from February 3rd to 6th at the Dubai World Trade Center, MEDLAB Middle East is one of the largest global exhibitions for laboratory and diagnostic professionals. NOUL was proud to stand alongside our industry leaders, demonstrating how our cutting-edge products deliver AI-powered diagnostics solutions that will reshape how we think of testing.
Indeed, the event held particular significance for NOUL as we recently received approval from the Saudi Food & Drug Authority (SFDA). This approval marks a key strategic milestone as we expand into the Middle East market.
MEDLAB Middle East 2025
 Source: MEDLAB
Source: MEDLAB
MEDLAB Middle East is a landmark event for anyone working in healthcare innovation and clinical pathology. With over 800 exhibitors and 20,000+ attendees from 180+ countries, this year’s event focused on technological advancements, automation, and sustainability in clinical pathology.
The diversity of presentations and workshops underscored the event’s regional (and global) significance. Companies and diagnostic professionals presented their insights into artificial intelligence (AI) and other digital technologies, exploring how they’re changing the diagnostic landscape.
As AI continues its inexorable march through the sector, NOUL represents the best-of-the-best, harnessing the power of AI to provide timely and accurate diagnostics. We engaged with healthcare providers, distributors, and government stakeholders from throughout the region, demonstrating how our platforms are perfect for addressing the region’s challenges and growing demand for advanced medical diagnostic tools.
miLab™ Diagnostic Solutions and Innovations
 Source: NOUL
Source: NOUL
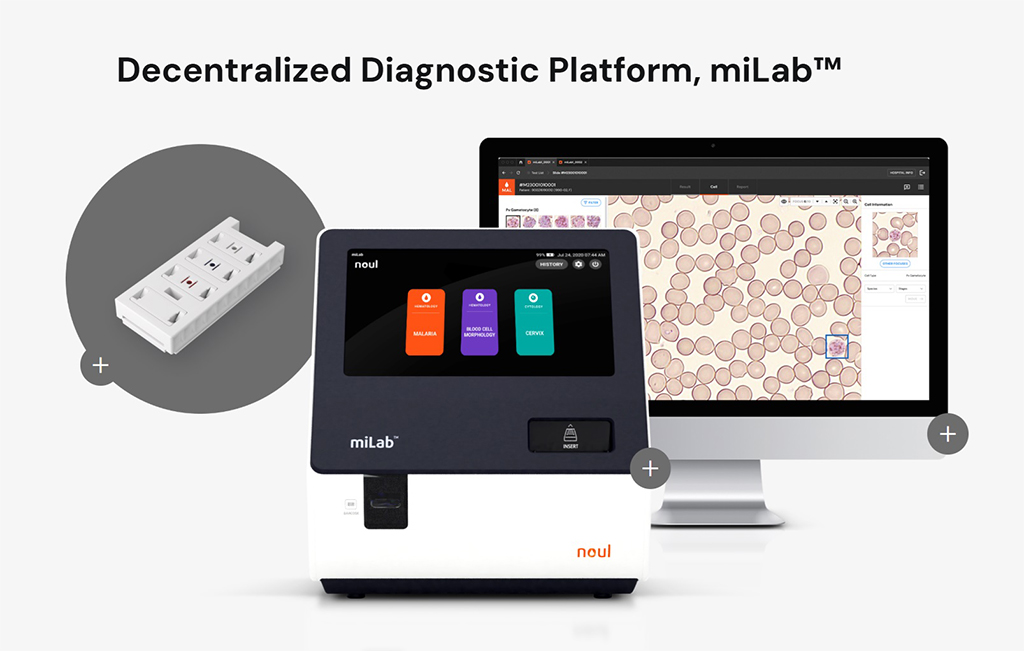
NOUL‘s premier technology is the miLab™, a compact medical diagnostic tool that relies on AI-powered technology to boost laboratory efficiency and accessibility. miLab™ integrates seamlessly into any healthcare setting, especially in regions with underdeveloped lab infrastructure.
The platform comes in several varieties:
- miLab™ MAL: A portable, AI-driven diagnostic tool tailored for malaria detection in resource-limited settings, ensuring rapid and reliable results.
- miLab™ BCM: Offers automated hematology testing, providing precise blood analysis through its advanced technology.
- miLab™ CER: Focuses on cervical cancer screening, emphasizing early detection and support for women’s health.
Each of these versions was built with scalability in mind. One of the principal problems globally is the inability to increase testing capacity rapidly. Current efforts rely on manual testing, where individuals must be trained to a high level to achieve reliable results. Governments and healthcare providers must also invest in advanced testing facilities.
miLab™ sidesteps these problems, minimizing the need for human intervention while enhancing diagnostic accuracy. So, whether a region is facing a malaria outbreak or is performing a cervical cancer screening program, only miLab™ has the potential to meet local demands.
In the past few years, NOUL has secured several key regulatory milestones in the Middle East, including:
- Received Medical Devices Marketing Authorization (MDMA) from the Saudi Food & Drug Authority (SFDA).
- The approval encompasses both the AI-powered software medical device (Class III) and its cartridge system, ensuring compliance with stringent Saudi regulatory standards.
- This SFDA approval is pivotal for miLab™’s expansion into the Middle East, with Saudi Arabia serving as a strategic hub for its growth across regional markets.
Engagement Opportunities at MEDLAB Middle East 2025
 Source: NOUL
Source: NOUL
One of the best parts of MEDLAB Middle East is the chance to meet and discuss new technologies with other professionals and stakeholders. NOUL showcased the miLab™ at the company’s booth (Z6.G50), attracting a high volume of visitors interested in its advanced diagnostic capabilities. On the first day alone, over 100 potential customers and partners visited the booth, with more than 40 meetings focused on potential partnerships. Significant inquiries came from key Middle Eastern markets such as Saudi Arabia, the UAE, and Kuwait, as well as from Asia and Africa. Attendees showed particular interest in miLab™ BCM for AI-driven blood analysis and miLab™ CER for cervical cytology, reflecting the growing demand for automated diagnostic solutions.
Each attendee experienced:
- Live demonstrations showcasing miLab™’s AI-powered diagnostics in action.
- One-on-one discussions with NOUL’s experts to explore tailored solutions for healthcare facilities.
- Presentations emphasizing miLab™’s role in improving diagnostic accuracy, speed, and affordability.
We aimed to provide a comprehensive introduction to the platform, allowing attendees to ask questions and gain deeper insights into miLab™. It’s all part of our “Do Less See More” campaign, addressing the needs of resource-limited healthcare environments across the Middle East and North Africa. miLab™ offers a powerful solution to enhance diagnostic efficiency while reducing workload.
miLab™‘s Commitment to Advancing Healthcare Innovation
 Source: NOUL
Source: NOUL
NOUL is already leading the way in AI-powered medical diagnostic tools. With miLab™, we’ve seen how healthcare workflows can be simplified without sacrificing the quantity of testing. We focus on automation and accessibility to ensure our solutions deliver what’s needed in regions facing critical shortages in laboratory resources.
Clinical pathology is crucial to modern medicine. But it depends too much on scarce resources and hard-to-acquire experts. miLab™ represents a paradigm shift in healthcare innovation. With new strategic partnerships with healthcare institutions and government agencies, we plan to expand access to our medical diagnostic tools throughout the Middle East, focusing on underserved areas. We’ll continue to invest in R&D efforts, pushing the possibilities for AI diagnostics.
Plus, as we take advantage of our new SFDA approval, we’ve got a golden opportunity to serve Saudi Arabia and the broader Middle Eastern market. It’s something we were pleased to hear again and again from the stakeholders we spoke to.
Conclusion

Source: NOUL
NOUL‘s participation in MEDLAB 2025 marked another step in the company’s push into the Middle Eastern market. Alongside SFDA approval, NOUL is providing a resounding success, driving engagement with key stakeholders and expanding its regional footprint.
As AI continues to evolve, we’re confident that NOUL will remain at the forefront of the industry. Harnessing the power of innovation, NOUL is set to redefine the future of diagnostics, ensuring that advanced healthcare solutions reach every corner of the globe.
If you’d like to learn more about NOUL, send us an inquiry. We’re more than happy to answer any questions.

