NOUL is revolutionizing malaria diagnostics with its AI-powered point-of-care testing devices. This commitment has driven the company to expand its efforts to combat malaria in West Africa, a region with a high malaria burden.
Two recent milestones highlight NOUL’s growing impact:
- The shipment of 55 miLab™ devices to Côte d’Ivoire.
- A public procurement contract valued at $4.7 million in Benin.
The Malaria Crisis in West Africa and the Need for Advanced Diagnostics
Source: Flickr
The fight against malaria in West Africa demands urgent attention, as this region bears a disproportionate share of the global malaria burden. In 2023, an estimated 249 million people worldwide were afflicted with malaria. Notably, countries such as Nigeria, Côte d’Ivoire, and Ghana rank among the hardest hit.
Nigeria alone reported approximately 67 million cases (27% of global cases) and accounted for 30.9% of worldwide malaria deaths. Meanwhile, Côte d’Ivoire recorded around 7.3 million cases (2.93% of global cases), and Ghana saw approximately 5.3 million cases (2.13%). These numbers illustrate why two-thirds of global malaria cases and deaths are concentrated in just 11 African countries, including Burkina Faso, Mali and Niger.
Beyond West Africa, nations such as Angola (8.4 million cases – 3.37% of global cases) and Kenya (3.4 million cases – 1.37%) also face widespread infections. Given these alarming statistics, the adoption of advanced malaria diagnostic test methodologies is critical to saving lives.
Chanllenges of Conventional Malaria Diagnostic Tests
Unfortunately, traditional diagnostic approaches come with significant limitations:
- Microscopy requires well-equipped laboratories and trained technicians—resources that are often scarce in remote areas.
- Rapid Diagnostic Tests (RDTs) can be unreliable, sometimes yielding false negatives, particularly in cases of P. falciparum infections with hrp2 gene deletions.
To learn more about the complexities of malaria detection, see Malaria Detection Insights from Prof. Beck.
These figures underscore the urgent need for innovative point-of-care testing devices to improve malaria detection and treatment outcomes.
Côte d’Ivoire: Expanding Malaria Diagnostics

Source: NOUL
On February 19th, NOUL shipped 55 miLab™ MAL devices to Côte d’Ivoire. This is an attempt to boost the nation’s capacity for accurate malaria diagnostic test administration. This delivery strengthens the local healthcare infrastructure and showcases the region’s growing confidence in advanced point-of-care testing devices.
Côte d’Ivoire remains a focal point in the West African malaria crisis. In fact, millions of cases are reported each year. The integration of AI-driven platforms into clinical settings allows the government to minimize diagnostic delays and significantly reduce mortality rates.
NOUL’s miLab™ MAL solution fits seamlessly with national health policies that target faster and more reliable malaria detection.
To learn how these innovations address gaps in rural healthcare, see Accurate Point-of-Care Testing Device and discover how new technologies can transform local disease management strategies.
Benin: A $4.7 Million Public Procurement Deal

Source: Freepik
Benin has also taken a proactive stance against malaria in West Africa. Recently, the country partnered with NOUL in a landmark public procurement contract that’s worth 6.34 billion won (approximately $4.7 million). Under the terms, NOUL will supply at least 219 miLab™ devices over the next three years, which marks the company’s first large-scale public procurement agreement with a national government.
Benin’s malaria burden includes about 5.1 million cases annually. The disease accounts for 40% of outpatient clinic visits and 25% of hospital admissions. These statistics highlight the urgent need for accurate and rapid malaria diagnostic test solutions, which NOUL’s AI-driven platforms aim to deliver.
Discover how cutting-edge diagnostic technologies are transforming malaria detection in 3 Key Solutions for Malaria Diagnosis.
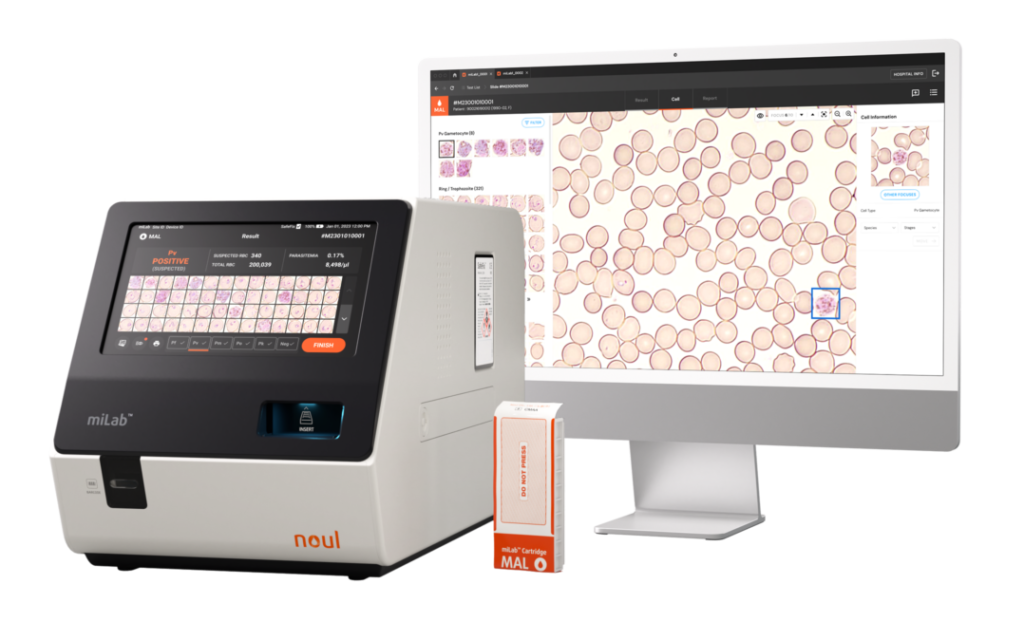
miLab™ MAL: A Globally Recognized Solution
Source: NOUL
NOUL’s miLab™ MAL has gained international recognition. Today, the company appears in UNITAID reports and earns medical device listings in multiple countries, including FDA listing. This track record attests to miLab™ MAL’s reliability in global health programs, especially when traditional methods are prone to human error or impeded by inadequate infrastructure. Unlike standard microscopy, miLab™ MAL combines digital imaging with AI, which performs a malaria diagnostic test that enhances both speed and accuracy.
Notably, the platform exhibits a 94.3% sensitivity rate for P. falciparum and 97.0% for P. vivax. Moreover, it demonstrates 98.1% accuracy for P. falciparum infections that feature hrp2 deletions (a common pitfall for RDTs). These performance metrics validate miLab™ MAL’s usefulness in public procurement deals, as governments can trust these point-of-care testing devices to operate effectively in diverse conditions.
Beyond its diagnostic excellence, the device’s AI-driven data analytics make it possible to perform real-time monitoring and rapid clinical decisions. This solution eliminates the heavy dependence on specialized personnel and laboratories.
For specifics on miLab™ MAL’s usage protocols, review NOUL miLab™ MAL Instructions for Use and see why it’s reshaping malaria in West Africa diagnostics.
Conclusion
As malaria in West Africa persists, innovative point-of-care testing devices like miLab™ MAL are becoming essential for healthcare systems combatting this endemic disease. With its AI-driven efficiency, reliability, and accessibility, NOUL’s platform directly addresses the region’s urgent need for precise malaria diagnostics.
Interested in partnering with NOUL or learning more about miLab™ MAL? Send us an inquiry to explore how our advanced diagnostic solutions can support your healthcare initiatives.

