
Source: noul
In July 2025, NOUL, an AI-driven diagnostic platform company, signed an MOU with Seegene Brazil, a global medical device manufacturer. Through this collaboration, NOUL plans to introduce miLab™ CER to the Brazilian market. Leveraging its experience and distribution network in Brazil’s diagnostic sector, Seegene Brazil aims to help expand the reach of NOUL’s cervical cancer diagnostic solution.
Following the MOU with Nihon Kohden Mexico in May, the partnership with Seegene Brazil further highlights the growing demand for innovative diagnostic technologies in the Latin American market. With the successful entry of both the blood test automation solution, miLab™ BCM, and the cervical cell screening solution, miLab™ CER, NOUL has established a strong foundation to set a new paradigm for AI-powered diagnostic automation across Latin America.
Cervical Cancer: The Third Most Common Cancer Among Women in Brazil
Source: Shutterstock
In Brazil, cervical cancer accounts for approximately 17,000 new cases annually, making it the third most common cancer among women. Both incidence and mortality rates remain higher than the global average, with a striking disparity between regions: in the north, the incidence reaches 20 cases per 100,000 women, compared to about 12.9 cases per 100,000 in the southeast.
Although Brazil operates a public HPV vaccination program, coverage remains relatively low at 57.7%. Pap screening is also irregular, relying largely on opportunistic testing, with less than 30% of eligible women undergoing screening. As a result, early detection of cervical cancer is limited, with few cases diagnosed at Stage 1. Many patients are only diagnosed once the cancer has already progressed, contributing to Brazil’s high incidence and mortality rates.
Persistent challenges such as regional disparities in healthcare access and limited public awareness have hindered progress in reducing cervical cancer rates. To address this, FEBRASGO (the Brazilian Federation of Gynecology and Obstetrics) launched a national campaign in 2023, highlighting the “90-70-90” approach: preventing cervical cancer with 90% vaccination coverage, ensuring at least 70% of eligible women are screened, and providing treatment for 90% of those diagnosed.
AI-Powered Diagnostic Solutions and Global Distribution Strength Driving Transformation

Source: Shutterstock
Brazil’s in IVD market, valued at approximately KRW 2.2 trillion, is the largest in Latin America and continues to grow rapidly. From the early stages of market entry, Seegene Brazil obtained regulatory approvals for 20 Allplex diagnostic products, including tests for sexually transmitted infections, respiratory diseases, and HPV, securing a strong foothold. The company now positions Brazil as a strategic hub for Latin America, driving expansion across the entire region.
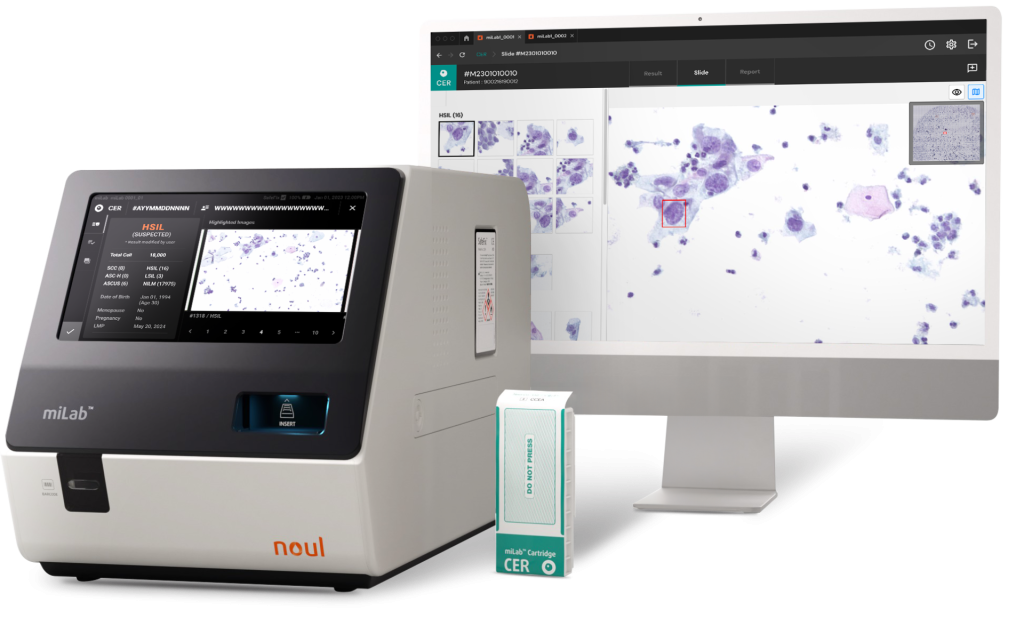
Noul’s miLab™ CER is entering this fast-growing diagnostic market with innovative technology at its core. Its solid-based staining cartridge automates the testing process, significantly simplifying workflows, while the proprietary on-device AI analyzes cervical cell images and delivers accurate, consistent results based on the Bethesda System (TBS) diagnostic criteria. Furthermore, in 2024, miLab™ CER was selected as one of the Top 3 WHO-recommended products by UNITAID for cervical cancer diagnostics—recognized alongside leading solutions from Roche and Hologic for its outstanding performance.
Seegene Brazil’s Strategic Move with miLab™ CER
Source: noul
The MOU between Noul and Seegene Brazil represents a strategic decision to combine Noul’s innovative AI diagnostic technology with Seegene’s expertise in the Brazilian market. Leveraging Seegene Brazil’s strong local presence and well-established distribution network, the partnership aims to expand access to advanced cervical cancer diagnostic solutions across the country.
Therefore, the AI-powered diagnostic automation platform, miLab™ CER, is expected to meet strong demand in Brazil’s in vitro diagnostics market, where the government is actively addressing cervical cancer prevention. Leveraging its outstanding performance, miLab™ CER is anticipated to enhance access to early cervical cancer detection, maximize diagnostic efficiency through precise and consistent results, and build trust with patients.
AI-Powered Diagnostic Innovation miLab™ CER Expanding Across Latin America

Source: noul
Recently, Noul presented miLab™ CER at the Seegene Brazil booth during the CBPC exhibition. The presentation highlighted the current challenges and status of cervical cancer in Brazil, sharing the diagnostic value that miLab™ CER can provide. In front of numerous stakeholders, the company emphasized the convenience offered by the all-in-one diagnostic platform and the high performance achieved through its innovative technology, presenting a vision to help close diagnostic gaps in the region and contribute to improving women’s health.
In March 2025, Noul signed agreements to supply miLab™ CER to six countries in Central America, including Panama, the Dominican Republic, and Nicaragua. With the recent decision to provide miLab™ CER to Seegene Brazil, Noul’s AI-powered cervical cancer solution is establishing a foundation for expansion across South America and is now entering a phase of active market growth.
Cervical Cancer: A Global Health Concern
To combat cervical cancer, recognized by the WHO as a global health concern, a “90-70-90” strategy has been proposed for 2030. Since early screening alone can dramatically reduce incidence and mortality rates, increasing screening coverage is a critical challenge. Encouragingly, demand for screening has been rising; however, a shortage of skilled personnel can lengthen the time from testing to diagnosis, reducing the likelihood of timely treatment.
Noul’s miLab™ CER addresses this challenge as an AI-powered solution that maximizes diagnostic efficiency. By enabling testing and diagnosis within the same workflow, it offers greater convenience for both healthcare professionals and patients. Moreover, by improving accessibility and delivering high-quality results, miLab™ CER has the potential to serve as a sustainable solution contributing to global health equity. Stay tuned to see how Noul’s technology can enhance the quality of healthcare services worldwide!
Learn more about Noul’s diagnostic automation platform, miLab™ CER.

