NOUL’s innovative miLab™ MAL system represents a new step in malaria diagnostics, using cutting-edge artificial intelligence (AI) to achieve very high accuracy in point-of-care testing.
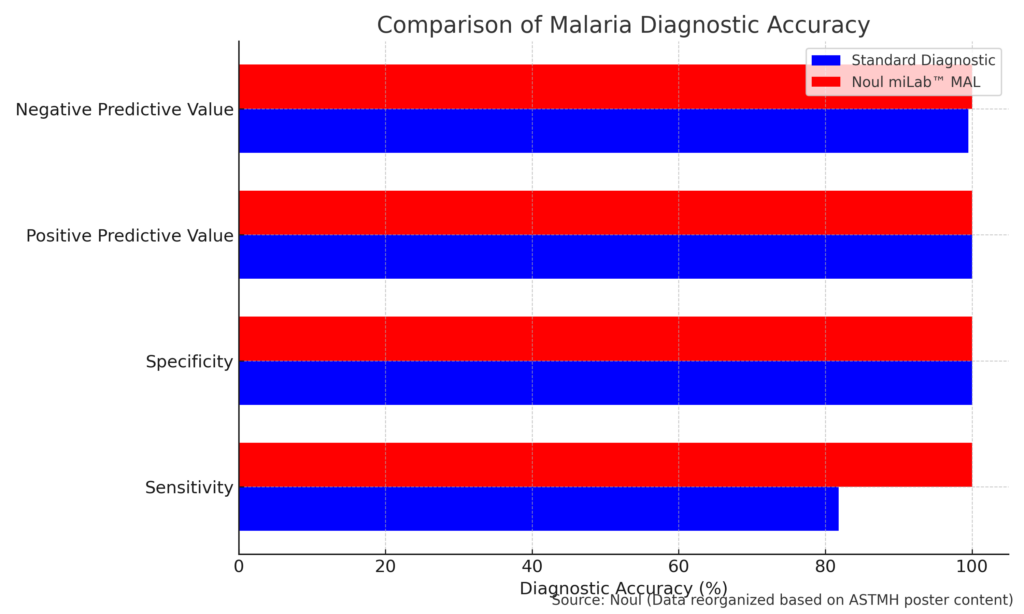
The accuracy of the device was tested in collaboration with Labcorp. Labcorp demonstrated 100% sensitivity, specificity, and predictive values. This precision will be critical for malaria-endemic regions where timely and accurate identification of the disease is vital. The AI-driven approach provides the possibility to revolutionize diagnostics through high speed, accuracy, and accessibility for healthcare providers globally.
Learn more about this technology at NOUL’s Official Page.
Why AI in Malaria Diagnosis is a Game Changer
Traditional methods to diagnose malaria (e.g., microscopy) require skilled personnel and are prone to human error. These techniques can be labor-intensive and time-consuming, especially in low-resource settings where diagnostic tools and trained professionals are limited. On the other hand, AI-driven diagnostics such as NOUL’s miLab™ MAL provide faster and more reliable results. This is thanks to the automation of the detection process and the analysis of blood samples with unmatched precision.
AI technology reduces errors that are inherent in manual microscopy and ensures consistent diagnostic performance. This tool is especially valuable in remote or underserved regions where healthcare resources are scarce. The integration of AI also allows for rapid scaling of diagnostic capabilities. This enables broader access to life-saving diagnostics in malaria-endemic and non-endemic areas.
Find additional insights about advancements in AI diagnostics on Korea Biomedical Review.
Point-of-Care Testing
 Source : Freepik
Source : Freepik
Point-of-care testing refers to diagnostic testing that’s performed at or near the site of patient care. This makes it possible to perform rapid clinical decision-making. Point-of-care testing ensures immediate results, which allows healthcare providers to act quickly and improve patient outcomes. Devices such as NOUL’s miLab™ MAL exemplify the advantages of point-of-care testing as they combine portability, accuracy, and advanced AI capabilities. The elimination of delays that are usually associated with centralized laboratories makes point-of-care testing valuable in remote or low-resource areas where quick malaria diagnosis is essential. For diseases like malaria, the speed and accuracy that’s provided by point-of-care testing can significantly reduce mortality rates and enhance treatment effectiveness.
Testing Success with Labcorp
NOUL’s partnership with Labcorp has validated the miLab™ MAL system through rigorous clinical trials. During these trials, the device achieved 100% accuracy in sensitivity, specificity, and predictive values, which surpasses traditional microscopy. Labcorp’s vast diagnostic network in the U.S. collected 409 blood samples. Of these samples, miLab™ MAL identified two additional positive cases that were initially missed by microscopy. These findings were later confirmed as accurate.
The collaboration with Labcorp demonstrates how NOUL is committed to make sure that its AI-powered technology meets the highest standards of reliability and performance. This success solidifies NOUL’s position as a leader in AI diagnostics and paves the way for its entry into the U.S. market.
How NOUL’s AI Technology Enhances Malaria Diagnosis in the U.S. and Beyond
Malaria cases in the U.S. rose during the past few years due to factors. Examples include global travel and climate change. Historically, most cases were imported. However, local transmission has become a concern. In fact, there are incidents reported in Texas, Florida, and Maryland.
As an FDA device listing, NOUL’s miLab™ MAL system provides a critical solution through rapid and accurate diagnoses that can improve patient outcomes and streamline healthcare responses. Its application is not limited to malaria-endemic regions but extends to areas where cases are rare. In order to the gap in diagnostic capabilities, NOUL’s technology presents a global solution to tackle malaria effectively. More information on malaria diagnostics can be found at the American Journal of Tropical Medicine and Hygiene.
The following table summarizes the accuracy of miLab™ MAL compared to other techniques:
How NOUL’s Participates in the Revolution of Global Healthcare
 Source : Shutterstock
Source : Shutterstock
Aside from malaria diagnostics, NOUL aims to expand its AI-powered platform to address other critical healthcare needs, such as blood analysis and cervical cancer detection. The company’s strategic vision focuses on becoming a global leader in diagnostic solutions through the power of AI to enhance accuracy, efficiency, and accessibility.
Today, NOUL’s efforts to enter the U.S. market are finally coming to light. There are plans to obtain FDA device listing Class 1 medical device approval and complete Laboratory Developed Test (LDT) certification by 2025. These milestones will allow the company to establish a strong presence in the U.S., which sets the stage for further global expansion. As NOUL continues to innovate, its commitment to improve global healthcare remains at the core of its mission. For further information on regulatory steps, explore FDA Device Listing.
Conclusion
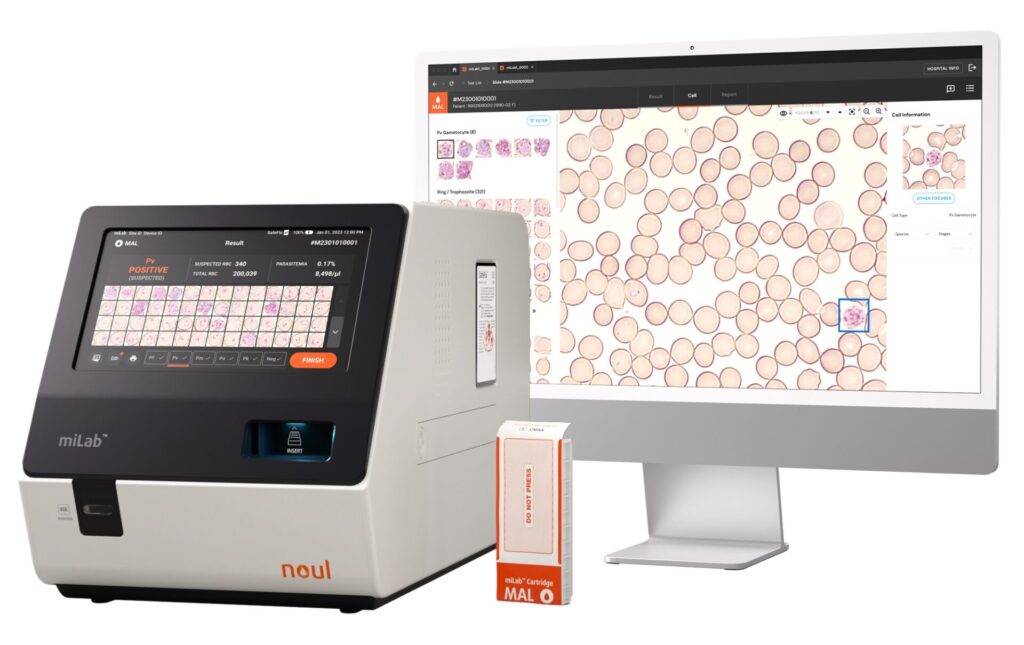 Source : NOUL
Source : NOUL
NOUL’s miLab™ MAL system is a prime example of the transformative potential of AI in healthcare. The latter will redefine the standards of malaria diagnostics. The combination of precision, speed, and accessibility makes this technology an extremely effective way to address critical healthcare challenges in endemic and non-endemic regions.
Check out this page for further details and inquiry about the product.